当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Template-Free Synthesis of Mesoporous β-MnO2 Nanoparticles: Structure, Formation Mechanism, and Catalytic Properties.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-07-30 , DOI: 10.1021/acsami.0c08043 Yui Yamaguchi 1 , Ryusei Aono 1 , Eri Hayashi 1 , Keigo Kamata 1 , Michikazu Hara 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-07-30 , DOI: 10.1021/acsami.0c08043 Yui Yamaguchi 1 , Ryusei Aono 1 , Eri Hayashi 1 , Keigo Kamata 1 , Michikazu Hara 1
Affiliation
|
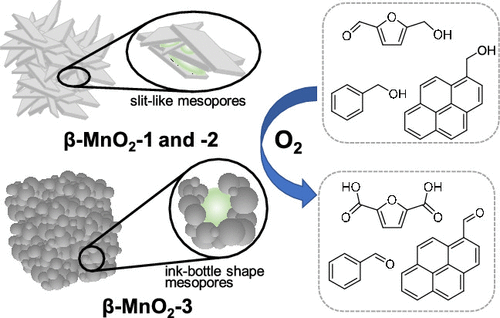
Mesoporous β-MnO2 nanoparticles were synthesized by a template-free low-temperature crystallization of Mn4+ precursors (low-crystallinity layer-type Mn4+ oxide, c-distorted H+-birnessite) produced by the reaction of MnO4– and Mn2+. The Mn starting materials, pH of the reaction solution, and calcination temperatures significantly affect the crystal structure, surface area, porous structure, and morphology of the manganese oxides formed. The pH conditions during the precipitation of Mn4+ precursors are important for controlling the morphology and porous structure of β-MnO2. Nonrigid aggregates of platelike particles with slitlike pores (β-MnO2-1 and -2) were obtained from the combinations of NaMnO4/MnSO4 and NaMnO4/Mn(NO3)2, respectively. On the other hand, spherelike particles with ink-bottle shaped pores (β-MnO2-3) were formed in NaMnO4/Mn(OAc)2 with pH adjustment (pH 0.8). The specific surface areas for β-MnO2-1, -2, and -3 were much higher than those for nonporous β-MnO2 nanorods synthesized using a typical hydrothermal method (β-MnO2-HT). On the other hand, c-distorted H+-birnessite precursors with a high interlayer metal cation (Na+ and K+) content led to the formation of α-MnO2 with a 2 × 2 tunnel structure. These mesoporous β-MnO2 materials acted as effective heterogeneous catalysts for the aerobic oxidation of 5-hydroxymethylfurfural (HMF) to 2,5-furandicarboxylic acid (FDCA) as a bioplastic monomer and for the transformation of aromatic alcohols to the corresponding aldehydes, where the catalytic activities of β-MnO2-1, -2, and -3 were approximately 1 order of magnitude higher than that of β-MnO2-HT. β-MnO2-3 exhibited higher catalytic activity (especially for larger molecules) than the other β-MnO2 materials, and this is likely attributed to the nanometer-sized spaces.
中文翻译:

无模板合成介孔β-MnO2纳米颗粒:结构,形成机理和催化性能。
介孔β-MnO的2个纳米颗粒的Mn无模板低温结晶合成4+前体(低结晶层型的Mn 4+氧化物,Ç -distortedħ + -birnessite)用MnO反应生成4 -和Mn 2+。Mn原料,反应溶液的pH值和煅烧温度显着影响所形成的锰氧化物的晶体结构,表面积,多孔结构和形态。Mn的沉淀过程中的pH条件4+前体是用于控制的形态和多孔结构β-MnO的重要2。与狭缝状板状粒子的聚集体非刚性孔(β -的MnO 2 - 1和- 2)从NaMnO的组合得到的4 /的MnSO 4和NaMnO 4 /锰(NO 3)2,分别。在另一方面,随着墨瓶形spherelike颗粒孔隙(β -的MnO 2 - 3)在NaMnO形成4 /锰(OAC)2与pH调节(pH为0.8)。比表面积为β -的MnO 2 - 1, - 2,以及- 3,均较非多孔β-MnO的高得多2个纳米棒的合成使用典型的水热法(β -的MnO 2 - HT)。在另一方面,Ç -distortedħ +具有高的层间金属阳离子-birnessite前体(钠+和K +)导致形成α-的MnO含量2与2×2隧道结构。这些孔β-的MnO 2充当有效的多相催化剂为5-羟甲基糠醛(HMF)的需氧氧化为2,5-呋喃二甲酸(FDCA),其为生物塑料单体和芳族醇的转化为相应的醛,其中的催化活性材料β -的MnO 2 - 1, - 2,和- 3分别约为1个数量级高于的β -的MnO 2 - HT。β -的MnO 2 - 3比其它β-MnO的表现出较高的催化活性(特别是对于较大分子)2 材料,这很可能归因于纳米级空间。
更新日期:2020-08-12
中文翻译:

无模板合成介孔β-MnO2纳米颗粒:结构,形成机理和催化性能。
介孔β-MnO的2个纳米颗粒的Mn无模板低温结晶合成4+前体(低结晶层型的Mn 4+氧化物,Ç -distortedħ + -birnessite)用MnO反应生成4 -和Mn 2+。Mn原料,反应溶液的pH值和煅烧温度显着影响所形成的锰氧化物的晶体结构,表面积,多孔结构和形态。Mn的沉淀过程中的pH条件4+前体是用于控制的形态和多孔结构β-MnO的重要2。与狭缝状板状粒子的聚集体非刚性孔(β -的MnO 2 - 1和- 2)从NaMnO的组合得到的4 /的MnSO 4和NaMnO 4 /锰(NO 3)2,分别。在另一方面,随着墨瓶形spherelike颗粒孔隙(β -的MnO 2 - 3)在NaMnO形成4 /锰(OAC)2与pH调节(pH为0.8)。比表面积为β -的MnO 2 - 1, - 2,以及- 3,均较非多孔β-MnO的高得多2个纳米棒的合成使用典型的水热法(β -的MnO 2 - HT)。在另一方面,Ç -distortedħ +具有高的层间金属阳离子-birnessite前体(钠+和K +)导致形成α-的MnO含量2与2×2隧道结构。这些孔β-的MnO 2充当有效的多相催化剂为5-羟甲基糠醛(HMF)的需氧氧化为2,5-呋喃二甲酸(FDCA),其为生物塑料单体和芳族醇的转化为相应的醛,其中的催化活性材料β -的MnO 2 - 1, - 2,和- 3分别约为1个数量级高于的β -的MnO 2 - HT。β -的MnO 2 - 3比其它β-MnO的表现出较高的催化活性(特别是对于较大分子)2 材料,这很可能归因于纳米级空间。































 京公网安备 11010802027423号
京公网安备 11010802027423号