当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bipolar Membrane Electrode Assemblies for Water Electrolysis
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-07-30 , DOI: 10.1021/acsaem.0c01127 Britta Mayerhöfer 1, 2 , David McLaughlin 1, 2 , Thomas Böhm 1, 2 , Manuel Hegelheimer 1, 2 , Dominik Seeberger 1, 2 , Simon Thiele 1, 2
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-07-30 , DOI: 10.1021/acsaem.0c01127 Britta Mayerhöfer 1, 2 , David McLaughlin 1, 2 , Thomas Böhm 1, 2 , Manuel Hegelheimer 1, 2 , Dominik Seeberger 1, 2 , Simon Thiele 1, 2
Affiliation
|
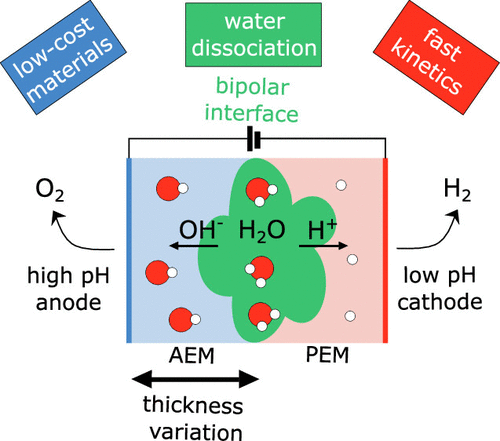
We present the first analysis of a zero-gap bipolar membrane water electrolyzer fed with liquid water. Our electrolyzers feature a high-pH environment for the oxygen evolution reaction and a low-pH environment for the hydrogen evolution reaction. The advantages of proton exchange membrane water electrolysis can be combined with those of anion exchange membrane water electrolysis by including a water splitting bipolar interface. First, we develop a KOH-free anion exchange membrane electrolysis cell. The cell’s alkaline anode serves as an integral building block on the path to a bipolar system. In a second step, we use this building block to investigate the cell operation characteristics of various cell configurations. We study the cell performance as the bipolar interface is shifted progressively toward the anode. A bipolar membrane with and without a water splitting catalyst resulted in cell current densities of 450 and 5 mA cm–2 at cell voltages of 2.2 V, respectively. Upon moving the bipolar interface directly between the acidic membrane and the high-pH anode, we achieved current densities of 9000 mA cm–2 at cell voltages of 2.2 V. Our study demonstrates the potential of this water electrolysis configuration, which should be adopted for further scientific studies and may show promise for future commercial water electrolysis systems.
中文翻译:

用于水电解的双极膜电极组件
我们介绍了一种零间隙双极性膜式电解水加液态水的首次分析。我们的电解槽在析氧反应中使用高pH环境,在析氢反应中使用低pH环境。质子交换膜水电解的优点可以通过包含水分解双极性界面而与阴离子交换膜水电解的优点相结合。首先,我们开发了无KOH阴离子交换膜电解槽。电池的碱性阳极是通往双极系统的必不可少的组成部分。在第二步中,我们使用此构造块来研究各种单元配置的单元操作特性。我们研究电池性能,因为双极界面逐渐移向阳极。电池电压分别为2.2 V时为–2。在酸性膜和高pH阳极之间直接移动双极界面时,在2.2 V的电池电压下,我们获得了9000 mA cm –2的电流密度。我们的研究证明了这种水电解配置的潜力,应将其用于进一步的科学研究,可能显示出未来商用水电解系统的前景。
更新日期:2020-07-30
中文翻译:

用于水电解的双极膜电极组件
我们介绍了一种零间隙双极性膜式电解水加液态水的首次分析。我们的电解槽在析氧反应中使用高pH环境,在析氢反应中使用低pH环境。质子交换膜水电解的优点可以通过包含水分解双极性界面而与阴离子交换膜水电解的优点相结合。首先,我们开发了无KOH阴离子交换膜电解槽。电池的碱性阳极是通往双极系统的必不可少的组成部分。在第二步中,我们使用此构造块来研究各种单元配置的单元操作特性。我们研究电池性能,因为双极界面逐渐移向阳极。电池电压分别为2.2 V时为–2。在酸性膜和高pH阳极之间直接移动双极界面时,在2.2 V的电池电压下,我们获得了9000 mA cm –2的电流密度。我们的研究证明了这种水电解配置的潜力,应将其用于进一步的科学研究,可能显示出未来商用水电解系统的前景。

































 京公网安备 11010802027423号
京公网安备 11010802027423号