背景与目标
肠上皮细胞 (IEC) 调节肠道免疫细胞,尤其是 T 辅助 17 (Th17) 细胞的发育。该过程的失调通过未知机制导致肠道炎症和肿瘤发生。TANK 结合激酶 1 (TBK1) 由 IEC 和先天免疫系统中的细胞表达。我们研究了 TBK1 在小鼠肠道免疫反应和肿瘤发生中的功能。
方法
我们进行野生型小鼠,小鼠的研究,有条件的破坏TBK1(TBK1 IEC-KO),TBK1 IEC-KO具有交叉小鼠装甲运兵最小/ +小鼠和山- / -小鼠越过装甲运兵最小/ +小鼠。一些小鼠接受了针对白细胞介素 17 (IL17) 或 IL1β 的中和抗体的腹腔注射。从小鼠收集肠组织并通过组织学分析腺瘤和 Th17 细胞的数量,并通过实时 PCR 分析炎性细胞因子的表达。从野生型和Tbk1 IEC-KO中分离出IEC 小鼠,用脂多糖刺激,与骨髓来源的巨噬细胞共培养,并通过 RNA 测序和生化分析进行分析。
结果
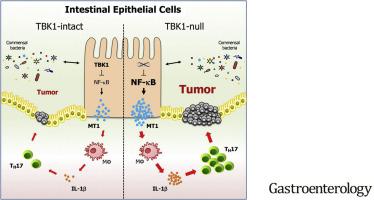
与Apc Min/+ Tbk1 WT小鼠相比,Apc Min/+ Tbk1 IEC-KO小鼠的肠息肉数量和大小显着增加,固有层中的 Th17 细胞显着增加。施用针对 IL17 的抗体使Apc Min/+ Tbk1 IEC-KO小鼠中肠息肉的数量减少到在Apc Min/+ Tbk1 WT小鼠中观察到的数量。在培养中,TBK1 缺陷型 IEC 促进巨噬细胞表达 IL1β,从而诱导幼稚 CD4 + 的分化T 细胞转化为 Th17 细胞。RNA 测序分析显示,TBK1 缺陷型 IEC 的金属硫蛋白 1 (MT1) 表达增加,金属硫蛋白 1 (MT1) 是一种促进肠道炎症的免疫调节剂。Apc Min/+ Mt –/–小鼠肠道组织中的 Th17 细胞明显少于Apc Min/+ Mt +/+小鼠,息肉数量也明显减少。癌症基因组图谱中结直肠肿瘤的分析发现,具有高水平MT1和IL17 mRNA 的结直肠肿瘤与患者生存时间缩短有关。
结论
IECs 对 TBK1 的表达抑制 MT1 的表达并阻止巨噬细胞表达 IL1β 和 Th17 细胞的分化,以防止炎症和肿瘤发生。可能会针对结直肠肿瘤的发生制定阻断该途径的策略。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Intestinal Epithelial TBK1 Prevents Differentiation of T-helper 17 Cells and Tumorigenesis in Mice.
Background & Aims
Intestinal epithelial cells (IECs) regulate intestinal immune cells, particularly development of T-helper 17 (Th17) cells. Deregulation of this process leads to intestinal inflammation and tumorigenesis, via unknown mechanisms. TANK-binding kinase 1 (TBK1) is expressed by IECs and cells in the innate immune system. We studied the functions of TBK1 in the intestinal immune response and tumorigenesis in mice.
Methods
We performed studies of wild-type mice, mice with conditional disruption of Tbk1 (Tbk1IEC-KO), Tbk1IEC-KO mice crossed with ApcMin/+ mice, and Mt–/– mice crossed with ApcMin/+ mice. Some mice were given intraperitoneal injections of a neutralizing antibody against interleukin 17 (IL17) or IL1β. Intestine tissues were collected from mice and analyzed by histology, for numbers of adenomas and Th17 cells, and expression of inflammatory cytokines by real-time PCR. IECs were isolated from wild-type and Tbk1IEC-KO mice, stimulated with lipopolysaccharide, co-cultured for with bone marrow-derived macrophages, and analyzed by RNA sequencing and biochemical analyses.
Results
Compared to ApcMin/+Tbk1WT mice, ApcMin/+Tbk1IEC-KO mice had significant increases in number and size of intestinal polyps, and significantly more Th17 cells in lamina propria. Administration of an antibody against IL17 reduced the number of intestinal polyps in ApcMin/+Tbk1IEC-KO mice to that observed in ApcMin/+Tbk1WT mice. In culture, TBK1-deficient IECs promoted expression of IL1β by macrophages, which induced differentiation of naïve CD4+ T cells into Th17 cells. RNA sequencing analysis revealed that the TBK1-deficient IECs had increased expression of metallothionein 1 (MT1), an immune regulator that promotes intestinal inflammation. Intestine tissues from ApcMin/+Mt–/– mice had significant fewer Th17 cells than ApcMin/+Mt+/+ mice, and a significantly lower number of polyps. Analyses of colorectal tumors in the Cancer Genome Atlas found colorectal tumors with high levels of MT1 and IL17 mRNAs to be associated with reduced survival times of patients.
Conclusions
Expression of TBK1 by IECs suppresses expression of MT1 and prevents expression of IL1β by macrophages and differentiation of Th17 cells, to prevent inflammation and tumorigenesis. Strategies to block this pathway might be developed for colorectal tumorigenesis.





















































 京公网安备 11010802027423号
京公网安备 11010802027423号