当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Evidence that the Solvent Barrier for Electron Transfer is Absent in the Electric Double Layer
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-30 , DOI: 10.1021/jacs.0c05226
Rachel E Bangle 1 , Jenny Schneider 1 , Daniel T Conroy 1 , Bruno M Aramburu-Trošelj 1 , Gerald J Meyer 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-30 , DOI: 10.1021/jacs.0c05226
Rachel E Bangle 1 , Jenny Schneider 1 , Daniel T Conroy 1 , Bruno M Aramburu-Trošelj 1 , Gerald J Meyer 1
Affiliation
|
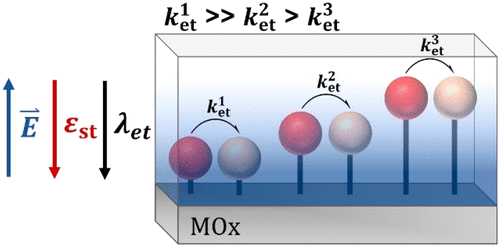
Classical capacitance studies have revealed that the first layer of water present at an aqueous metal-electrolyte interface has a die-lectric constant less than 1/10th that of bulk water. Modern theory indicates that under such solvent conditions the barrier for elec-tron transfer will decrease substantially, yet this important prediction has not been tested experimentally. Here we report interfa-cial electron transfer kinetics for molecules positioned at variable distanced within the electric double layer of a transparent con-ductive oxide as a function of the Gibbs free energy change. The data indicate that the solvent reorganization is indeed near zero and increases to bulk values only when the molecules are positioned greater than 15 Å from the conductive electrode. Consistent with this conclusion, lateral intermolecular electron transfer, parallel to a semiconducting oxide electrode, was shown to be more rapid when the molecules were within the electric double layer. The results provide much needed feedback for theoretical studies and also indicate a huge kinetic advantage for aqueous electron transfer and redox catalysis that takes place proximate to a solid interface.
中文翻译:

双电层中不存在用于电子转移的溶剂屏障的动力学证据
经典电容研究表明,存在于水性金属-电解质界面的第一层水的介电常数小于本体水的介电常数的 1/10。现代理论表明,在这种溶剂条件下,电子转移的势垒将大大降低,但这一重要预测尚未经过实验验证。在这里,我们报告了位于透明导电氧化物双电层内可变距离的分子的界面电子转移动力学,作为吉布斯自由能变化的函数。数据表明,溶剂重组确实接近于零,并且仅当分子位于距导电电极 15 埃以上时才会增加到体积值。与这一结论一致,横向分子间电子转移,与半导体氧化物电极平行,当分子在双电层内时,速度更快。结果为理论研究提供了急需的反馈,并且还表明在固体界面附近发生的水性电子转移和氧化还原催化具有巨大的动力学优势。
更新日期:2020-07-30
中文翻译:

双电层中不存在用于电子转移的溶剂屏障的动力学证据
经典电容研究表明,存在于水性金属-电解质界面的第一层水的介电常数小于本体水的介电常数的 1/10。现代理论表明,在这种溶剂条件下,电子转移的势垒将大大降低,但这一重要预测尚未经过实验验证。在这里,我们报告了位于透明导电氧化物双电层内可变距离的分子的界面电子转移动力学,作为吉布斯自由能变化的函数。数据表明,溶剂重组确实接近于零,并且仅当分子位于距导电电极 15 埃以上时才会增加到体积值。与这一结论一致,横向分子间电子转移,与半导体氧化物电极平行,当分子在双电层内时,速度更快。结果为理论研究提供了急需的反馈,并且还表明在固体界面附近发生的水性电子转移和氧化还原催化具有巨大的动力学优势。

































 京公网安备 11010802027423号
京公网安备 11010802027423号