Physica E: Low-dimensional Systems and Nanostructures ( IF 2.9 ) Pub Date : 2020-07-30 , DOI: 10.1016/j.physe.2020.114377
Sittipranee Ploychompoo , Qianwei Liang , Xin Zhou , Chen Wei , Hanjin Luo
|
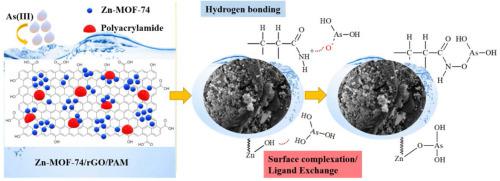
Contamination of drinking water with heavy metals, particularly arsenic (As), is a persistent problem with serious public health implications worldwide. In this study, we present a zinc based metal-organic framework (Zn-MOF-74) and polyacrylamide polymer (PAM) coated on reduced graphene oxide (rGO) as an effective adsorbent for the removal of arsenite (As(III)) from water. Zn-MOF-74 nanoparticles were prepared by room-temperature precipitation and these were immobilized on rGO surface grafted PAM by a free-radical polymerization method, (Zn-MOF-74/rGO/PAM nanocomposites). The experimental data correlates well with the pseudo-second-order kinetic model and Langmuir isotherm, and the maximum adsorption capacity (qmax) was 282.4 mg g−1 at pH 10, 298 K. The removal efficiency was rapid, removing more than 99.8% of As(III) from a 0.2 mg L−1 solution and achieving drinkable levels in 15 min. Thermodynamic data revealed that the process was spontaneous and endothermic. Furthermore, the adsorbent revealed high stability in pH range 4–10 and could be reused at least four times. Adsorption mechanism involved a synergistic combination of chemisorption and physisorption. FTIR and XPS analyzes revealed that the amide group (–NH2) and hydroxyl group (–OH) on Zn-MOF-74/rGO/PAM dominate in their adsorption.
中文翻译:

Zn-MOF-74 /聚丙烯酰胺涂层的还原氧化石墨烯(Zn-MOF-74 / rGO / PAM)的制备,用于去除As(III)
饮用水被重金属特别是砷污染是一个持续存在的问题,在世界范围内对公共卫生产生了严重影响。在这项研究中,我们提出了一种锌基金属有机骨架(Zn-MOF-74)和聚丙烯酰胺聚合物(PAM),该聚合物涂覆在还原的氧化石墨烯(rGO)上,作为一种有效的吸附剂,用于从中去除砷(As(III))。水。Zn-MOF-74纳米颗粒通过室温沉淀法制备,并通过自由基聚合方法(Zn-MOF-74 / rGO / PAM纳米复合材料)固定在rGO表面接枝的PAM上。实验数据与拟二级动力学模型和Langmuir等温线密切相关,最大吸附容量(q max)为282.4 mg g -1 在pH 10时为298K。去除效率很快,从0.2 mg L -1溶液中去除了99.8%以上的As(III),并在15分钟内达到了可饮用的水平。热力学数据表明该过程是自发的且吸热的。此外,该吸附剂在4-10的pH范围内显示出高稳定性,可以重复使用至少四次。吸附机理涉及化学吸附和物理吸附的协同结合。FTIR和XPS分析表明,Zn-MOF-74 / rGO / PAM上的酰胺基(–NH 2)和羟基(–OH)在其吸附中占主导地位。

































 京公网安备 11010802027423号
京公网安备 11010802027423号