当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the Regioselectivity of Hydroheteroarylation of Allylbenzene with Pyridine Catalyzed by Ni/AlMe3 with N-Heterocyclic Carbene: The Concerted Hydrogen Transfer Mechanism.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-07-29 , DOI: 10.1021/acs.joc.0c01449 Phiphob Naweephattana 1 , Boodsarin Sawatlon 1 , Panida Surawatanawong 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-07-29 , DOI: 10.1021/acs.joc.0c01449 Phiphob Naweephattana 1 , Boodsarin Sawatlon 1 , Panida Surawatanawong 1, 2
Affiliation
|
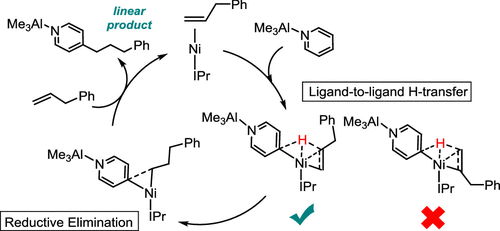
The hydroheteroarylation of allylbenzene with pyridine as catalyzed by Ni/AlMe3 and a N-heterocyclic carbene ligand has recently been established. Density functional calculations revealed that the common stepwise pathway, which involves the C–H oxidative addition of pyridine-AlMe3 before the migratory insertion of allylbenzene, is unlikely as the migratory insertion needs to overcome a prohibitively high energy barrier. In contrast, the ligand-to-ligand hydrogen transfer pathway is more favorable in which the hydrogen is transferred directly from the para-position of pyridine-AlMe3 to C2 of allylbenzene. Our distortion–interaction analysis and natural bond orbital analysis indicate that the interaction energy is strongly correlated with the extent of the charge transfer from the alkene (hydrogen acceptor) to the pyridine-AlMe3 (hydrogen donor), which dictates the selectivity of the H-transfer to the C2 position of allylbenzene. Then, the subsequent C–C reductive elimination of the regioselective linear product is facilitated by the steric hindrance of the IPr ligand. Understanding these key factors affecting the product regioselectivity is important to the development of catalysts for hydroheteroarylation of alkenes.
中文翻译:

Ni / AlMe3与N-杂环碳烯催化烯丙基苯与吡啶的氢杂芳基化的区域选择性的见解:协同的氢转移机理。
最近已经建立了由Ni / AlMe 3和N-杂环卡宾配体催化的烯丙基苯与吡啶的氢杂芳基化。密度泛函计算表明,在逐步迁移烯丙基苯之前,涉及到吡啶-AlMe 3的C–H氧化加成反应的常见逐步途径不太可能,因为迁移途径需要克服极高的能垒。相反,配体到配体的氢转移途径是更有利的,其中氢直接从吡啶-AlMe 3的对位转移烯丙基苯的C2。我们的畸变-相互作用分析和自然键轨道分析表明,相互作用能与从烯烃(氢受体)到吡啶-AlMe 3(氢供体)的电荷转移程度密切相关,这决定了氢的选择性-转移至烯丙基苯的C 2位置。然后,IPr配体的空间位阻促进了随后的C–C区域选择性线性产物的还原还原。了解这些影响产物区域选择性的关键因素对于烯烃加氢杂芳基化催化剂的开发很重要。
更新日期:2020-09-05
中文翻译:

Ni / AlMe3与N-杂环碳烯催化烯丙基苯与吡啶的氢杂芳基化的区域选择性的见解:协同的氢转移机理。
最近已经建立了由Ni / AlMe 3和N-杂环卡宾配体催化的烯丙基苯与吡啶的氢杂芳基化。密度泛函计算表明,在逐步迁移烯丙基苯之前,涉及到吡啶-AlMe 3的C–H氧化加成反应的常见逐步途径不太可能,因为迁移途径需要克服极高的能垒。相反,配体到配体的氢转移途径是更有利的,其中氢直接从吡啶-AlMe 3的对位转移烯丙基苯的C2。我们的畸变-相互作用分析和自然键轨道分析表明,相互作用能与从烯烃(氢受体)到吡啶-AlMe 3(氢供体)的电荷转移程度密切相关,这决定了氢的选择性-转移至烯丙基苯的C 2位置。然后,IPr配体的空间位阻促进了随后的C–C区域选择性线性产物的还原还原。了解这些影响产物区域选择性的关键因素对于烯烃加氢杂芳基化催化剂的开发很重要。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号