当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Systematic Study of Methylation from Benzene to Hexamethylbenzene in H-SSZ-13 Using Density Functional Theory and Ab Initio Calculations
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-07-28 , DOI: 10.1021/acscatal.0c02037 Michal Fečík 1 , Philipp N. Plessow 1 , Felix Studt 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-07-28 , DOI: 10.1021/acscatal.0c02037 Michal Fečík 1 , Philipp N. Plessow 1 , Felix Studt 1, 2
Affiliation
|
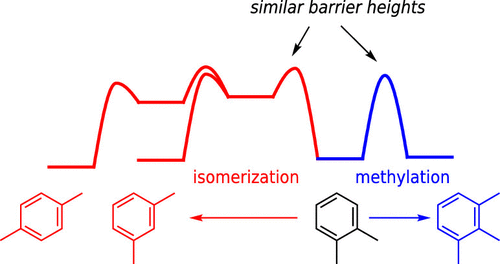
Methylation of aromatic molecules in H-SSZ-13 is studied from benzene to hexamethylbenzene via all possible isomers. Both methylations with methanol (MeOH) and dimethyl ether (DME) are investigated via both stepwise and concerted mechanisms. Calculations are carried out using periodic density functional theory corrected by high-level DLPNO-CCSD(T) calculations on cluster models. While we find little selectivity between the methylations of different aromatics, our calculations indicate that isomerization via methyl shifts between different isomers is a viable mechanism with barriers comparable to methylation. The generally observed trend is that MeOH methylation barriers are smaller than those using DME, with the difference between MeOH and DME increasing with the level of methylation of the aromatic molecule.
中文翻译:

利用密度泛函理论和从头算计算系统研究H-SSZ-13中苯甲基化为六甲基苯
通过所有可能的异构体研究了H-SSZ-13中芳族分子的甲基化,从苯到六甲基苯。通过逐步和协同机理研究了甲醇(MeOH)和二甲醚(DME)的甲基化。计算是使用周期性密度泛函理论进行的,该理论通过对聚类模型的高级DLPNO-CCSD(T)计算进行了修正。虽然我们发现不同芳族化合物的甲基化之间几乎没有选择性,但我们的计算表明,通过不同异构体之间的甲基转移进行异构化是可行的机制,其屏障与甲基化相当。通常观察到的趋势是,与使用DME的情况相比,MeOH的甲基化势垒要小,而MeOH和DME之间的差异会随着芳族分子的甲基化程度而增加。
更新日期:2020-08-08
中文翻译:

利用密度泛函理论和从头算计算系统研究H-SSZ-13中苯甲基化为六甲基苯
通过所有可能的异构体研究了H-SSZ-13中芳族分子的甲基化,从苯到六甲基苯。通过逐步和协同机理研究了甲醇(MeOH)和二甲醚(DME)的甲基化。计算是使用周期性密度泛函理论进行的,该理论通过对聚类模型的高级DLPNO-CCSD(T)计算进行了修正。虽然我们发现不同芳族化合物的甲基化之间几乎没有选择性,但我们的计算表明,通过不同异构体之间的甲基转移进行异构化是可行的机制,其屏障与甲基化相当。通常观察到的趋势是,与使用DME的情况相比,MeOH的甲基化势垒要小,而MeOH和DME之间的差异会随着芳族分子的甲基化程度而增加。

































 京公网安备 11010802027423号
京公网安备 11010802027423号