Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
X 射线光电子能谱研究结晶和非晶氧化铝氮化形成的氮的化学键
RSC Advances ( IF 3.9 ) Pub Date : 2020-7-28 , DOI: 10.1039/d0ra05104g
K Lawniczak-Jablonska 1 , Z R Zytkiewicz 1 , S Gieraltowska 1 , M Sobanska 1 , P Kuzmiuk 1 , K Klosek 1
Affiliation
|
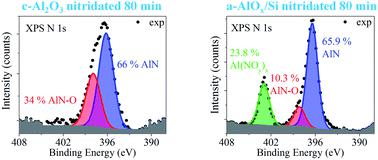
人们已经做出了许多努力来优化晶体蓝宝石(c-Al 2 O 3 )衬底的氮化,而对非晶氧化铝层(a-AlO x )的氮化却很少关注。因此,需要对非晶氧化铝薄膜与氮物质的反应进行广泛分析,以阐明氮掺入此类层的机制并控制其性能。在这项工作中,X 射线光电子能谱用于确定氮等离子体处理 c-Al 2 O 3和通过原子层沉积在硅和蓝宝石衬底上生长的 15 nm 厚 a-AlO x层而形成的氮的化学态。结果表明,c-Al 2 O 3和a-AlO x样品的氮化过程显着不同,我们将其与两种材料的初始化学计量相关联。在蓝宝石表面发现了 O 空位,这是通过氮有限扩散取代氧形成 AlN 型键合所必需的。这个过程很缓慢并且涉及氮氧化物AlN-O的形成。 80 分钟氮化后,仅掺入了约 3.4 at% 的 N。相反,在a-AlO x层中,氮化之前存在Al空位。 这开辟了一条新的、更有效的氮掺入途径,即通过在缺阳离子晶格中积累氮并形成 Al(NO y ) x相,然后形成 AlN 和 AlN-O。正如实际观察到的那样,这种情况预测 a-AlO x中的氮掺入比 c-Al 2 O 3更有效。这也解释了我们的发现:由于蓝宝石衬底提供了氧,硅上的 a-AlO x中的氮含量比蓝宝石上的氮含量要多。

"点击查看英文标题和摘要"







































 京公网安备 11010802027423号
京公网安备 11010802027423号