当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photo-induced preparation of unnatural α-amino acids: synthesis and characterization of novel Leu5-enkephalin analogues
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-07-28 , DOI: 10.1039/d0qo00696c Hongxiang Xue 1, 2, 3, 4, 5 , Mengzhun Guo 3, 4, 6, 7, 8 , Chao Wang 3, 4, 6, 7, 8 , Yuxuan Shen 1, 2, 3, 4, 5 , Rupeng Qi 3, 4, 6, 7, 8 , Yifei Wu 1, 2, 3, 4, 5 , Zhaoqing Xu 3, 4, 6, 7, 8 , Min Chang 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-07-28 , DOI: 10.1039/d0qo00696c Hongxiang Xue 1, 2, 3, 4, 5 , Mengzhun Guo 3, 4, 6, 7, 8 , Chao Wang 3, 4, 6, 7, 8 , Yuxuan Shen 1, 2, 3, 4, 5 , Rupeng Qi 3, 4, 6, 7, 8 , Yifei Wu 1, 2, 3, 4, 5 , Zhaoqing Xu 3, 4, 6, 7, 8 , Min Chang 1, 2, 3, 4, 5
Affiliation
|
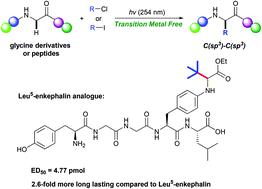
We here report the first example of the photo-induced preparation of unnatural α-amino acids and modification of peptides with unactivated alkyl chlorides (iodides) without the assistance of a transition metal catalyst or photosensitizer. The method was successfully applied for the late-stage modification of peptides and provided a novel enkephalin analogue with significantly better analgesic activity and 2.6-fold more long-lasting time compared to its parent peptide.
中文翻译:

光诱导的非天然α-氨基酸的制备:新型Leu5-脑啡肽类似物的合成与表征
我们在这里报告了第一个例子,说明了在不借助过渡金属催化剂或光敏剂的情况下,光诱导制备非天然α-氨基酸和用未活化的烷基氯(碘化物)修饰肽的方法。该方法已成功应用于肽段的后期修饰,并提供了一种新型脑啡肽类似物,其镇痛活性明显优于其母体肽,并且持久时间延长了2.6倍。
更新日期:2020-08-25
中文翻译:

光诱导的非天然α-氨基酸的制备:新型Leu5-脑啡肽类似物的合成与表征
我们在这里报告了第一个例子,说明了在不借助过渡金属催化剂或光敏剂的情况下,光诱导制备非天然α-氨基酸和用未活化的烷基氯(碘化物)修饰肽的方法。该方法已成功应用于肽段的后期修饰,并提供了一种新型脑啡肽类似物,其镇痛活性明显优于其母体肽,并且持久时间延长了2.6倍。































 京公网安备 11010802027423号
京公网安备 11010802027423号