当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of Furin Protease Binding to SARS-CoV-2 Spike Glycoprotein and Implications for Potential Targets and Virulence.
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2020-07-28 , DOI: 10.1021/acs.jpclett.0c01698 Naveen Vankadari 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2020-07-28 , DOI: 10.1021/acs.jpclett.0c01698 Naveen Vankadari 1
Affiliation
|
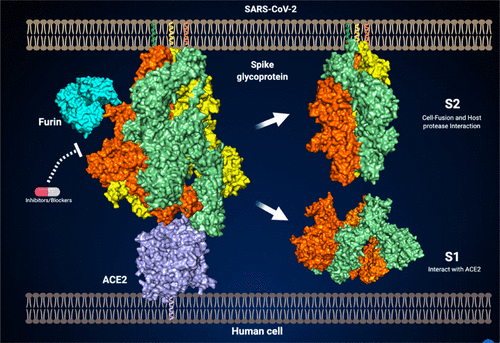
The COVID-19 pandemic is an urgent global health emergency, and the presence of Furin site in the SARS-CoV-2 spike glycoprotein alters virulence and warrants further molecular, structural, and biophysical studies. Here we report the structure of Furin in complex with SARS-CoV-2 spike glycoprotein, demonstrating how Furin binds to the S1/S2 region of spike glycoprotein and eventually cleaves the viral protein using experimental functional studies, molecular dynamics, and docking. The structural studies underline the mechanism and mode of action of Furin, which is a key process in host cell entry and a hallmark of enhanced virulence. Our whole-exome sequencing analysis shows the genetic variants/alleles in Furin were found to alter the binding affinity for viral spike glycoprotein and could vary in infectivity in humans. Unravelling the mechanisms of Furin action, binding dynamics, and the genetic variants opens the growing arena of bona fide antibodies and development of potential therapeutics targeting the blockage of Furin cleavage.
中文翻译:

弗林蛋白酶与 SARS-CoV-2 刺突糖蛋白结合的结构及其对潜在靶点和毒力的影响。
COVID-19 大流行是全球紧急卫生事件,SARS-CoV-2 刺突糖蛋白中弗林蛋白酶位点的存在改变了毒力,需要进一步的分子、结构和生物物理学研究。在这里,我们报告了弗林蛋白酶与 SARS-CoV-2 刺突糖蛋白复合物的结构,展示了弗林蛋白酶如何与刺突糖蛋白的 S1/S2 区域结合,并通过实验功能研究、分子动力学和对接最终裂解病毒蛋白。结构研究强调了弗林蛋白酶的作用机制和模式,弗林蛋白酶是进入宿主细胞的关键过程,也是毒力增强的标志。我们的全外显子组测序分析显示,弗林蛋白酶中的遗传变异/等位基因可以改变病毒刺突糖蛋白的结合亲和力,并且可能改变人类的感染性。解开弗林蛋白酶的作用机制、结合动力学和遗传变异,开启了真正抗体的不断发展的领域,并开发了针对阻断弗林蛋白酶裂解的潜在疗法。
更新日期:2020-08-20
中文翻译:

弗林蛋白酶与 SARS-CoV-2 刺突糖蛋白结合的结构及其对潜在靶点和毒力的影响。
COVID-19 大流行是全球紧急卫生事件,SARS-CoV-2 刺突糖蛋白中弗林蛋白酶位点的存在改变了毒力,需要进一步的分子、结构和生物物理学研究。在这里,我们报告了弗林蛋白酶与 SARS-CoV-2 刺突糖蛋白复合物的结构,展示了弗林蛋白酶如何与刺突糖蛋白的 S1/S2 区域结合,并通过实验功能研究、分子动力学和对接最终裂解病毒蛋白。结构研究强调了弗林蛋白酶的作用机制和模式,弗林蛋白酶是进入宿主细胞的关键过程,也是毒力增强的标志。我们的全外显子组测序分析显示,弗林蛋白酶中的遗传变异/等位基因可以改变病毒刺突糖蛋白的结合亲和力,并且可能改变人类的感染性。解开弗林蛋白酶的作用机制、结合动力学和遗传变异,开启了真正抗体的不断发展的领域,并开发了针对阻断弗林蛋白酶裂解的潜在疗法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号