Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrowetting of Hydrofluoroether Liquid Droplet at a Gold Electrode/Water Interface: Significance of Lower Adhesion Energy and Static Friction Energy.
Langmuir ( IF 3.7 ) Pub Date : 2020-07-27 , DOI: 10.1021/acs.langmuir.0c00829 Tetsuro Morooka 1 , Takamasa Sagara 2
Langmuir ( IF 3.7 ) Pub Date : 2020-07-27 , DOI: 10.1021/acs.langmuir.0c00829 Tetsuro Morooka 1 , Takamasa Sagara 2
Affiliation
|
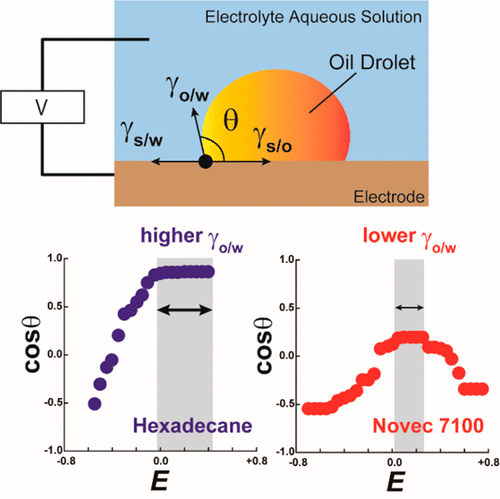
We explored the electrowetting behavior of a hydrofluoroester solvent, Novec 7100 (Novec), as a liquid droplet on a Au(111) electrode in water (0.05 M KClO4). Comparison with the electrowetting of hexadecane (HD) highlighted the significance of the lower adhesion energy and static friction energy of Novec than those of HD. The electrode potential-dependent contact angle θ of a Novec droplet showed little hysteresis. When potentials were set by means of potential steps, a Novec droplet increased its θ at more positive potentials than the potential of zero charge (pzc) of the Au(111) electrode. We found that the key factor of the electrowetting behavior for Novec is its low adhesion energy and static friction energy. The static friction energy of the oils to the Au(111) electrode surface was evaluated by a comparative analysis of the potential dependence of the interfacial tension at the solid/water interface, ΔγS/W–E curve, calculated from electrochemical surface charge data and the experimental cos θ–E curve: 2.6 mN/m for HD and 0.95 mN/m for Novec. When Br– was added in the aqueous solution to allow its adsorption on the Au surface surrounding a Novec droplet, the potential of maximum cos θ was shifted to negative. Overall, although the Novec droplet showed a narrower range of θ change than a HD droplet, the Novec droplet seldom got stuck to the surface as far as potential step was used, reflecting the narrower plateau region of θ near the pzc. Also, the specific adsorption of a coexistent anion was a significant factor of θ. This work has featured the significance of a slippy droplet on an electrode surface, giving an impact on the technology of microfluid transportation control by electric potentials.
中文翻译:

氢氟醚液滴在金电极/水界面处的电润湿:降低附着力和静态摩擦能的意义。
我们探索了氢氟酸酯溶剂Novec 7100(Novec)在水中的Au(111)电极上的液滴(0.05 M KClO 4)。与十六烷(HD)的电润湿比较表明,Novec的粘附能和静摩擦能比HD低,这很重要。Novec液滴的电极电位依赖性接触角θ几乎没有磁滞现象。当通过电位阶跃设置电位时,Novec液滴在比Au(111)电极的零电荷(pzc)电位更高的正电位下增加了θ。我们发现,Novec电润湿行为的关键因素是其低粘附能和静摩擦能。通过对固/水界面上界面张力的电位依赖性ΔγS / W – E的比较分析,评估了油对Au(111)电极表面的静摩擦能。由电化学表面电荷数据和实验cosθ- E曲线计算得出的曲线:HD为2.6 mN / m,Novec为0.95 mN / m。当Br –在水溶液中加入N 2 O 3使其吸附在Novec液滴周围的Au表面上,最大cosθ的电势变为负。总体而言,尽管Novec液滴的θ变化范围比HD液滴的窄,但只要使用电位阶跃,Novec液滴很少会粘附在表面上,从而反映了pzc附近θ的较窄的平稳区域。另外,共存阴离子的比吸附是θ的重要因素。这项工作的特点是在电极表面上形成光滑液滴的重要性,这对通过电势控制微流体传输的技术产生了影响。
更新日期:2020-08-25
中文翻译:

氢氟醚液滴在金电极/水界面处的电润湿:降低附着力和静态摩擦能的意义。
我们探索了氢氟酸酯溶剂Novec 7100(Novec)在水中的Au(111)电极上的液滴(0.05 M KClO 4)。与十六烷(HD)的电润湿比较表明,Novec的粘附能和静摩擦能比HD低,这很重要。Novec液滴的电极电位依赖性接触角θ几乎没有磁滞现象。当通过电位阶跃设置电位时,Novec液滴在比Au(111)电极的零电荷(pzc)电位更高的正电位下增加了θ。我们发现,Novec电润湿行为的关键因素是其低粘附能和静摩擦能。通过对固/水界面上界面张力的电位依赖性ΔγS / W – E的比较分析,评估了油对Au(111)电极表面的静摩擦能。由电化学表面电荷数据和实验cosθ- E曲线计算得出的曲线:HD为2.6 mN / m,Novec为0.95 mN / m。当Br –在水溶液中加入N 2 O 3使其吸附在Novec液滴周围的Au表面上,最大cosθ的电势变为负。总体而言,尽管Novec液滴的θ变化范围比HD液滴的窄,但只要使用电位阶跃,Novec液滴很少会粘附在表面上,从而反映了pzc附近θ的较窄的平稳区域。另外,共存阴离子的比吸附是θ的重要因素。这项工作的特点是在电极表面上形成光滑液滴的重要性,这对通过电势控制微流体传输的技术产生了影响。

































 京公网安备 11010802027423号
京公网安备 11010802027423号