Cell Reports ( IF 7.5 ) Pub Date : 2020-07-28 , DOI: 10.1016/j.celrep.2020.107974
Fang Zhang 1 , Pengyi Yan 1 , Huijing Yu 1 , Huangying Le 1 , Zixuan Li 1 , Jiahuan Chen 1 , Xiaodong Liang 1 , Shiyan Wang 2 , Weiting Wei 1 , Li Liu 1 , Yan Zhang 3 , Xing Ji 1 , Anyong Xie 4 , Wantao Chen 5 , Zeguang Han 1 , William T Pu 6 , Sun Chen 1 , Yingwei Chen 1 , Kun Sun 1 , Baoxue Ge 7 , Bing Zhang 1
|
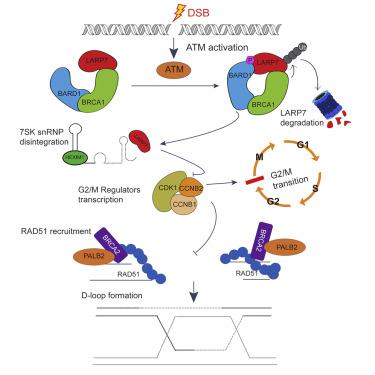
Attenuated DNA repair leads to genomic instability and tumorigenesis. BRCA1/BARD1 are the best-known tumor suppressors that promote homology recombination (HR) and arrest cell cycle. However, it remains ambiguous whether and how their E3 ligase activity regulates HR. Here, we demonstrate that upon genotoxic stress, BRCA1 together with BARD1 catalyzes the K48 polyubiquitination on LARP7, a 7SK RNA binding protein known to control RNAPII pausing, and thereby degrades it through the 26S ubiquitin-proteasome pathway. Depleting LARP7 suppresses the expression of CDK1 complex, arrests the cell at the G2/M DNA damage checkpoint, and reduces BRCA2 phosphorylation, which thereby facilitates RAD51 recruitment to damaged DNA to enhance HR. Importantly, LARP7 depletion observed in breast cancer patients leads to chemoradiotherapy resistance both in vitro and in vivo. Altogether, this study unveils a mechanism by which BRCA1/BARD1 control HR and cell cycle, and highlights LARP7 as a potential target for cancer prevention and therapy.
中文翻译:

L ARP7是BRCA1泛素酶底物,可调节基因组稳定性和肿瘤发生。
弱化的DNA修复导致基因组不稳定和肿瘤发生。BRCA1 / BARD1是最知名的肿瘤抑制因子,可促进同源重组(HR)和阻滞细胞周期。但是,它们的E3连接酶活性是否以及如何调节HR仍不清楚。在这里,我们证明了在遗传毒性胁迫下,BRCA1与BARD1一起催化LARP7(一种已知可控制RNAPII暂停的7SK RNA结合蛋白)上的K48多聚泛素化作用,并因此通过26S泛素-蛋白酶体途径降解它。耗尽LARP7可抑制CDK1复合物的表达,将细胞停在G2 / M DNA损伤检查点,并减少BRCA2磷酸化,从而促进RAD51募集到受损的DNA从而增强HR。重要的是,在乳腺癌患者中观察到的LARP7耗竭会导致放化疗耐药体外和体内。总而言之,这项研究揭示了BRCA1 / BARD1控制HR和细胞周期的机制,并强调了LARP7作为癌症预防和治疗的潜在靶标。

































 京公网安备 11010802027423号
京公网安备 11010802027423号