Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-07-28 , DOI: 10.1016/j.bioorg.2020.104144
Valentina Loconte 1 , Michele Cianci 2 , Ilaria Menozzi 3 , Davide Sbravati 3 , Francesco Sansone 3 , Alessandro Casnati 3 , Rodolfo Berni 3
|
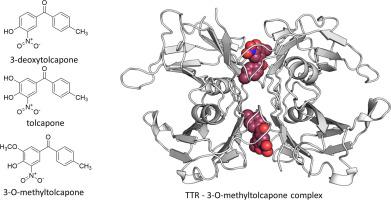
运甲状腺素蛋白(TTR)是一种淀粉样蛋白生成的同型四聚体,参与血液和脑脊液中甲状腺素和视黄醇的运输。TTR稳定剂,例如tolcapone,一种FDA批准的帕金森氏病药物,能够与TTR的甲状腺素结合位点的残基(无论是野生型还是致病性突变体形式)相互作用,从而稳定其四聚体天然状态并抑制淀粉样蛋白生成。在本文中,我们报道了新型的TTR稳定剂3-deoxytolcapone的合成。进行了3-O-甲基甲苯酮和3-脱氧甲苯酮与TTR相互作用的高分辨率X射线分析。在两个TTR-配体复合物中,托卡朋类似物主要与TTR四聚体的甲状腺素结合位点的残基建立H键和疏水相互作用。两种化合物在存在血浆蛋白的情况下均具有较高的TTR选择性稳定性,尽管它们的“正向”和“反向”结合方式分别明显不同。实际上,与二甲苯二聚体-二聚体界面处建立的新的相互作用补偿了与三苯乙酮和3-O-甲基甲苯二酮相比,与3-脱氧苯乙酮的蛋白质残基的稳定相互作用的丧失或减弱。我们的数据,加上先前报道的托卡朋和3-O-甲基甲苯酮的人体内药代动力学特性数据,进一步支持了后者托卡酮类似物作为TTR稳定剂的相关性。与二甲苯二聚体-二聚体界面上建立的新的相互作用补偿了与三乙酮和3-O-甲基甲苯二酮相比,与3-脱氧甲苯酮的蛋白质残基的稳定相互作用的丧失或减弱。我们的数据,加上先前报道的托卡朋和3-O-甲基托卡朋在人体内药代动力学特性的数据,进一步支持了后者托卡朋类似物作为TTR稳定剂的相关性。与二甲苯二聚体-二聚体界面上建立的新的相互作用补偿了与三乙酮和3-O-甲基甲苯二酮相比,与3-脱氧甲苯酮的蛋白质残基的稳定相互作用的丧失或减弱。我们的数据,加上先前报道的托卡朋和3-O-甲基托卡朋在人体内药代动力学特性的数据,进一步支持了后者托卡朋类似物作为TTR稳定剂的相关性。

"点击查看英文标题和摘要"




































 京公网安备 11010802027423号
京公网安备 11010802027423号