Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Micellization Behavior of Conventional Cationic Surfactants within Glycerol-Based Deep Eutectic Solvent.
ACS Omega ( IF 3.7 ) Pub Date : 2020-07-27 , DOI: 10.1021/acsomega.0c00866 Ramesh Kumar Banjare 1 , Manoj Kumar Banjare 1, 2 , Kamalakanta Behera 3 , Siddharth Pandey 4 , Kallol K Ghosh 2
ACS Omega ( IF 3.7 ) Pub Date : 2020-07-27 , DOI: 10.1021/acsomega.0c00866 Ramesh Kumar Banjare 1 , Manoj Kumar Banjare 1, 2 , Kamalakanta Behera 3 , Siddharth Pandey 4 , Kallol K Ghosh 2
Affiliation
|
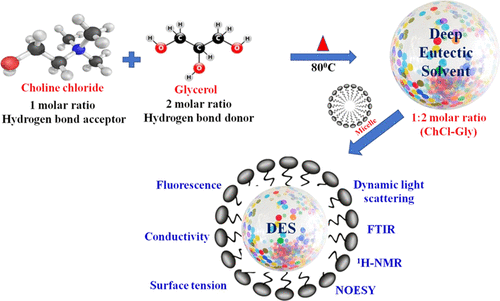
The aggregation behavior of two cationic surfactants, i.e., cetyldimethylethanolammonium bromide (CDMEAB) and cetyltributylphosphonium bromide (CTBPB), within an aqueous deep eutectic solvent (DES) is studied. The synthesized DES is composed of 1:2 mole ratio of choline chloride and glycerol and is further characterized by Fourier transform infrared (FTIR) and 1H NMR spectroscopy techniques. The critical micellar concentration (CMC), micellar size, and intermolecular interaction in surfactants within Gly-based DES solutions are investigated by various techniques including surface tension, conductivity, fluorescence, dynamic light scattering (DLS), FTIR, 1H NMR, and two-dimensional (2D) nuclear Overhauser effect spectroscopy (NOESY). The various interfacial properties and thermodynamic parameters are determined in the presence of 5 wt % glyceline (Gly)-based DES in an aqueous solution. The CMC, aggregation number (Nagg), and Stern–Volmer constant (Ksv) have also been determined by a steady-state fluorescence method. DLS is used to obtain information regarding the size of the aggregates formed by the cationic surfactants in DES solutions. FTIR spectroscopy is used to study the surfactant–DES interactions that tune the micellar structure of the surfactants within the Gly-based DES solution. The functional groups involved in the interactions (H-bonding and electrostatic) are the head groups (HO–CH2–CH2–N+ ion for CDMEAB and quaternary phosphonium (P+) ion for CTBPB) of the surfactants with the −OH-containing Gly DES. The hydrophobic moieties are involved in the hydrophobic interactions. The 1H NMR data show that differences in chemical shifts can provide significant information about the interactions taking place within the system. 1H NMR and NOESY techniques are further employed to strengthen our claim on the feasible structural arrangements within the aqueous surfactant–DES self-assembled structures. It is observed that both the cationic surfactants, i.e., CDMEAB and CTBPB, form self-assembled nanostructures in the Gly-based DES solutions. The present results are expected to be useful for colloidal solutions of DES and their mixtures with water.
中文翻译:

常规阳离子表面活性剂在甘油基低共熔溶剂中的胶束化行为。
研究了两种阳离子表面活性剂,即十六烷基二甲基乙醇溴化铵(CDMEAB)和十六烷基三丁基溴化鏻(CTBPB)在水性低共熔溶剂(DES)中的聚集行为。合成的 DES 由摩尔比为 1:2 的氯化胆碱和甘油组成,并通过傅里叶变换红外 (FTIR) 和1 H NMR 光谱技术进一步表征。通过各种技术研究基于 Gly 的 DES 溶液中表面活性剂的临界胶束浓度 (CMC)、胶束尺寸和分子间相互作用,包括表面张力、电导率、荧光、动态光散射 (DLS)、FTIR、 1 H NMR 和两种技术。维(2D)核欧沃豪塞效应光谱(NOESY)。各种界面性质和热力学参数是在水溶液中存在 5 wt% 甘油 (Gly) 基 DES 的情况下测定的。 CMC、聚集数 ( N agg ) 和 Stern-Volmer 常数 ( K sv ) 也已通过稳态荧光方法测定。 DLS 用于获取有关 DES 溶液中阳离子表面活性剂形成的聚集体尺寸的信息。 FTIR 光谱用于研究表面活性剂与 DES 的相互作用,从而调节基于甘氨酸的 DES 溶液中表面活性剂的胶束结构。参与相互作用(氢键和静电)的官能团是具有 -OH 的表面活性剂的头部基团(对于 CDMEAB 为 HO–CH 2 –CH 2 –N +离子,对于 CTBPB 为季磷鎓 (P + ) 离子) -含有甘氨酸DES。疏水部分参与疏水相互作用。 1 H NMR 数据表明,化学位移的差异可以提供有关系统内发生的相互作用的重要信息。进一步采用1 H NMR 和 NOESY 技术来加强我们对水性表面活性剂-DES 自组装结构中可行结构排列的主张。据观察,两种阳离子表面活性剂,即 CDMEAB 和 CTBPB,在基于 Gly 的 DES 溶液中形成自组装纳米结构。目前的结果预计可用于 DES 的胶体溶液及其与水的混合物。
更新日期:2020-08-11
中文翻译:

常规阳离子表面活性剂在甘油基低共熔溶剂中的胶束化行为。
研究了两种阳离子表面活性剂,即十六烷基二甲基乙醇溴化铵(CDMEAB)和十六烷基三丁基溴化鏻(CTBPB)在水性低共熔溶剂(DES)中的聚集行为。合成的 DES 由摩尔比为 1:2 的氯化胆碱和甘油组成,并通过傅里叶变换红外 (FTIR) 和1 H NMR 光谱技术进一步表征。通过各种技术研究基于 Gly 的 DES 溶液中表面活性剂的临界胶束浓度 (CMC)、胶束尺寸和分子间相互作用,包括表面张力、电导率、荧光、动态光散射 (DLS)、FTIR、 1 H NMR 和两种技术。维(2D)核欧沃豪塞效应光谱(NOESY)。各种界面性质和热力学参数是在水溶液中存在 5 wt% 甘油 (Gly) 基 DES 的情况下测定的。 CMC、聚集数 ( N agg ) 和 Stern-Volmer 常数 ( K sv ) 也已通过稳态荧光方法测定。 DLS 用于获取有关 DES 溶液中阳离子表面活性剂形成的聚集体尺寸的信息。 FTIR 光谱用于研究表面活性剂与 DES 的相互作用,从而调节基于甘氨酸的 DES 溶液中表面活性剂的胶束结构。参与相互作用(氢键和静电)的官能团是具有 -OH 的表面活性剂的头部基团(对于 CDMEAB 为 HO–CH 2 –CH 2 –N +离子,对于 CTBPB 为季磷鎓 (P + ) 离子) -含有甘氨酸DES。疏水部分参与疏水相互作用。 1 H NMR 数据表明,化学位移的差异可以提供有关系统内发生的相互作用的重要信息。进一步采用1 H NMR 和 NOESY 技术来加强我们对水性表面活性剂-DES 自组装结构中可行结构排列的主张。据观察,两种阳离子表面活性剂,即 CDMEAB 和 CTBPB,在基于 Gly 的 DES 溶液中形成自组装纳米结构。目前的结果预计可用于 DES 的胶体溶液及其与水的混合物。













































 京公网安备 11010802027423号
京公网安备 11010802027423号