Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of CO2 conversion into methanol and methane at the edge of graphitic carbon nitride sheet: A first-principle study
Carbon ( IF 10.5 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.carbon.2020.07.006 Hong-Zhang Wu , Sateesh Bandaru , Jin Liu , Li-Li Li , Lin Jin
Carbon ( IF 10.5 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.carbon.2020.07.006 Hong-Zhang Wu , Sateesh Bandaru , Jin Liu , Li-Li Li , Lin Jin
|
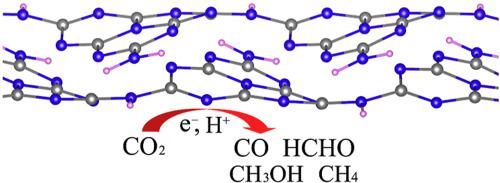
Abstract Rational conversion of CO2 into valuable fuels would partially or fully close the carbon loop and can have significant environmental benefits. The elemental steps of CO2 conversion into hydrocarbon fuels such as methanol, methane and a series intermediates at the edge of graphitic carbon nitride were investigated using first-principle calculations. Herein, we reported the detailed mechanism of CO2 reduction at the edge of g-C3N4 sheet including two possible pathways based on H-shuttling model. The participation of hydrogen within NH group at the edge of g-C3N4 sheet as another hydrogen source could promote the hydrogenation of intermediate through the rearrangement reaction. Methanol is a quite favorable product than methane during CO2 reduction reaction. Carbon monoxide (CO) and formaldehyde as the products would limit the conversion of CO2 to hydrocarbon fuels.
中文翻译:

石墨氮化碳片边缘处CO2转化为甲醇和甲烷的机理:第一性原理研究
摘要 将 CO2 合理转化为有价值的燃料将部分或完全关闭碳循环,并具有显着的环境效益。使用第一性原理计算研究了 CO2 转化为碳氢化合物燃料(如甲醇、甲烷和石墨氮化碳边缘的一系列中间体)的元素步骤。在此,我们报告了 g-C3N4 片边缘 CO2 还原的详细机制,包括基于 H 穿梭模型的两种可能途径。g-C3N4片层边缘的NH基团中的氢作为另一个氢源的参与可以通过重排反应促进中间体的氢化。在 CO2 还原反应中,甲醇是比甲烷更有利的产物。
更新日期:2020-11-01
中文翻译:

石墨氮化碳片边缘处CO2转化为甲醇和甲烷的机理:第一性原理研究
摘要 将 CO2 合理转化为有价值的燃料将部分或完全关闭碳循环,并具有显着的环境效益。使用第一性原理计算研究了 CO2 转化为碳氢化合物燃料(如甲醇、甲烷和石墨氮化碳边缘的一系列中间体)的元素步骤。在此,我们报告了 g-C3N4 片边缘 CO2 还原的详细机制,包括基于 H 穿梭模型的两种可能途径。g-C3N4片层边缘的NH基团中的氢作为另一个氢源的参与可以通过重排反应促进中间体的氢化。在 CO2 还原反应中,甲醇是比甲烷更有利的产物。































 京公网安备 11010802027423号
京公网安备 11010802027423号