当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atomically Dispersed Ru Catalyst for Low-Temperature Nitrogen Activation to Ammonia via an Associative Mechanism
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-07-24 , DOI: 10.1021/acscatal.0c00549 Xiuyun Wang 1 , Lingling Li 1 , Zhongpu Fang 2 , Yongfan Zhang 2 , Jun Ni 1 , Bingyu Lin 1 , Lirong Zheng 3 , Chak-tong Au 1 , Lilong Jiang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-07-24 , DOI: 10.1021/acscatal.0c00549 Xiuyun Wang 1 , Lingling Li 1 , Zhongpu Fang 2 , Yongfan Zhang 2 , Jun Ni 1 , Bingyu Lin 1 , Lirong Zheng 3 , Chak-tong Au 1 , Lilong Jiang 1
Affiliation
|
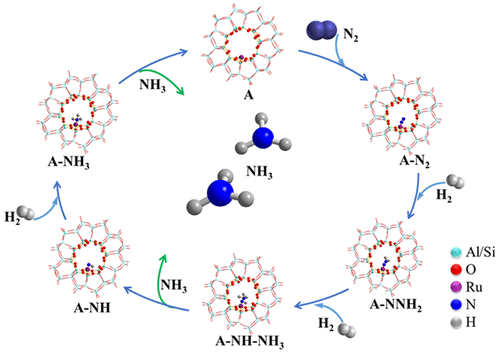
The industrial synthesis of NH3 using Fe- or Ru-based catalysts usually requires harsh reaction conditions. It is desirable to develop catalysts that perform well at low temperature and pressure (250–400 °C, <2 MPa). The main challenge of low-temperature NH3 synthesis is the dissociation of the extremely stable N≡N triple bond. Herein, we report the design of homogeneous single-atom Ru centers on an H-ZMS-5 (HZ) support with the Ru atoms individually anchored in the micropores of HZ, effectively boosting NH3 synthesis under mild conditions via an associative pathway. Synchrotron-based near-edge X-ray absorption fine structure (NEXAFS) and in situ DRIFTS analyses show that ═N– groups are the primary intermediates, and DFT calculations further show that, unlike Ru nanoclusters, the cooperation of a single Ru atom and hydrogen species in HZ leads to N2 hydrogenation rather than direct N2 dissociation, and the indirect N–N bond dissociation occurs much more easily via the formation of the NHNH3* intermediate; the energy barrier for breaking the N–N bond keeps falling from 2.90 eV for *N2 to 0.04 eV for *NHNH3, showing that N2 hydrogenation is an effective way for sharp weakening of N–N bonds. Moreover, the rate-determining step is shifted from the dissociation of the N≡N triple bond to the formation of *N2H2. As a consequence, the single-atom 0.2 wt % Ru/H-ZSM-5 catalyst shows the highest NH3 synthesis rate per gram of Ru (1.26 molNH3 gRu–1 h–1 at 300 °C and 1 MPa) among the Ru-based catalysts ever reported.
中文翻译:

原子分散的Ru催化剂通过关联机制将低温氮活化为氨
使用基于铁或钌的催化剂的NH 3的工业合成通常需要苛刻的反应条件。希望开发出在低温和低压(250-400°C,<2 MPa)下表现良好的催化剂。低温NH 3合成的主要挑战是极其稳定的N≡N三键的离解。在本文中,我们报告了在H-ZMS-5(HZ)载体上设计的均质单原子Ru中心,其中Ru原子分别锚定在HZ的微孔中,在温和条件下通过缔合途径有效地促进了NH 3的合成。基于同步加速器的近边缘X射线吸收精细结构(NEXAFS)和原位DRIFTS分析表明,═N–基团是主要中间体,DFT计算进一步表明,与Ru纳米团簇不同,HZ中单个Ru原子和氢物种的协同作用导致N 2氢化而不是直接N 2解离,并且间接NH键解离通过NHNH 3 *中间体的形成更容易发生。破坏N–N键的能垒从* N 2的2.90 eV下降到* NHNH 3的0.04 eV ,表明N 2氢化是显着削弱N–N键的有效方法。此外,速率确定步骤从N≡N三键的解离转移到* N 2的形成H 2。结果,在0.2重量%Ru / H-ZSM-5单原子催化剂中,每克Ru的NH 3合成率最高(在300°C和1 MPa时为1.26 mol NH3 g Ru –1 h –1)。曾经报道过钌基催化剂。
更新日期:2020-08-21
中文翻译:

原子分散的Ru催化剂通过关联机制将低温氮活化为氨
使用基于铁或钌的催化剂的NH 3的工业合成通常需要苛刻的反应条件。希望开发出在低温和低压(250-400°C,<2 MPa)下表现良好的催化剂。低温NH 3合成的主要挑战是极其稳定的N≡N三键的离解。在本文中,我们报告了在H-ZMS-5(HZ)载体上设计的均质单原子Ru中心,其中Ru原子分别锚定在HZ的微孔中,在温和条件下通过缔合途径有效地促进了NH 3的合成。基于同步加速器的近边缘X射线吸收精细结构(NEXAFS)和原位DRIFTS分析表明,═N–基团是主要中间体,DFT计算进一步表明,与Ru纳米团簇不同,HZ中单个Ru原子和氢物种的协同作用导致N 2氢化而不是直接N 2解离,并且间接NH键解离通过NHNH 3 *中间体的形成更容易发生。破坏N–N键的能垒从* N 2的2.90 eV下降到* NHNH 3的0.04 eV ,表明N 2氢化是显着削弱N–N键的有效方法。此外,速率确定步骤从N≡N三键的解离转移到* N 2的形成H 2。结果,在0.2重量%Ru / H-ZSM-5单原子催化剂中,每克Ru的NH 3合成率最高(在300°C和1 MPa时为1.26 mol NH3 g Ru –1 h –1)。曾经报道过钌基催化剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号