当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Lipid Activation Mechanism of a Transmembrane Potassium Channel
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-23 , DOI: 10.1021/jacs.0c01991 Collin G Borcik 1 , Derek B Versteeg 1 , Reza Amani 1 , Maryam Yekefallah 1 , Nazmul H Khan 1 , Benjamin J Wylie 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-23 , DOI: 10.1021/jacs.0c01991 Collin G Borcik 1 , Derek B Versteeg 1 , Reza Amani 1 , Maryam Yekefallah 1 , Nazmul H Khan 1 , Benjamin J Wylie 1
Affiliation
|
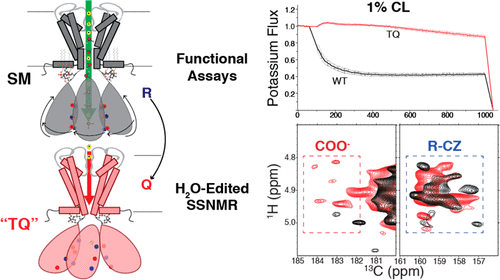
Membrane proteins and lipids coevolved to yield unique co-regulatory mechanisms. Inward-rectifier K+ (Kir) channels are often activated by anionic lipids endemic to their native membranes and require accessible water along their K+ conductance pathway. To better understand Kir channel activation, we target multiple mutants of the Kir channel KirBac1.1 via solid state Nuclear Magnetic Resonance (SSNMR) spectroscopy, potassium efflux assays, and Förster-resonance-energy-transfer (FRET) measurements. In the I131C stability mutant (SM), we observe an open-active channel in the presence of anionic lipids with greater activity upon addition of cardiolipin (CL). Introducing three R to Q mutations (R49/151/153Q (TQ)) renders the protein inactive within the same activating lipid environment. Our SSNMR experiments reveal a stark reduction of lipid-protein interactions in the TQ mutant explaining the dramatic loss of channel activity. Water-edited SSNMR experiments further determined the TQ mutant possesses greater overall solvent exposure in comparison to wild type, but with reduced water accessibility along the ion conduction pathway, consistent with the closed state of the channel. These experiments also suggest water is proximal to the selectivity filter of KirBac1.1 in the open-activated state, but that it may not directly enter the selectivity filter. Our findings suggest lipid binding initiates a concerted rotation of the cytoplasmic domain subunits, which is stabilized by multiple inter-subunit salt bridges. This action buries ionic side chains away from the bulk water, while allowing water greater access to the K+ conduction pathway. This work highlights universal membrane protein motifs, including lipid-protein interactions, domain rearrangement, and water-mediated diffusion mechanisms.
中文翻译:

跨膜钾通道的脂质激活机制
膜蛋白和脂质共同进化以产生独特的共同调节机制。内向整流 K+ (Kir) 通道通常被其天然膜特有的阴离子脂质激活,并且需要沿其 K+ 电导途径获得水。为了更好地了解 Kir 通道激活,我们通过固态核磁共振 (SSNMR) 光谱、钾外流测定和 Förster 共振能量转移 (FRET) 测量来定位 Kir 通道 KirBac1.1 的多个突变体。在 I131C 稳定性突变体 (SM) 中,我们在阴离子脂质存在下观察到一个开放的活性通道,在添加心磷脂 (CL) 后活性更高。引入三个 R 到 Q 突变(R49/151/153Q (TQ))使蛋白质在相同的激活脂质环境中失活。我们的 SSNMR 实验揭示了 TQ 突变体中脂质-蛋白质相互作用的明显减少,解释了通道活性的显着丧失。水编辑 SSNMR 实验进一步确定,与野生型相比,TQ 突变体具有更大的整体溶剂暴露,但沿离子传导途径的水可及性降低,与通道的关闭状态一致。这些实验还表明,水在开放激活状态下靠近 KirBac1.1 的选择性过滤器,但它可能不会直接进入选择性过滤器。我们的研究结果表明,脂质结合启动了细胞质结构域亚基的协同旋转,这由多个亚基间盐桥稳定。这个动作使离子侧链远离大量水,同时允许水更多地进入 K+ 传导通路。这项工作突出了通用的膜蛋白基序,包括脂蛋白相互作用、结构域重排和水介导的扩散机制。
更新日期:2020-07-23
中文翻译:

跨膜钾通道的脂质激活机制
膜蛋白和脂质共同进化以产生独特的共同调节机制。内向整流 K+ (Kir) 通道通常被其天然膜特有的阴离子脂质激活,并且需要沿其 K+ 电导途径获得水。为了更好地了解 Kir 通道激活,我们通过固态核磁共振 (SSNMR) 光谱、钾外流测定和 Förster 共振能量转移 (FRET) 测量来定位 Kir 通道 KirBac1.1 的多个突变体。在 I131C 稳定性突变体 (SM) 中,我们在阴离子脂质存在下观察到一个开放的活性通道,在添加心磷脂 (CL) 后活性更高。引入三个 R 到 Q 突变(R49/151/153Q (TQ))使蛋白质在相同的激活脂质环境中失活。我们的 SSNMR 实验揭示了 TQ 突变体中脂质-蛋白质相互作用的明显减少,解释了通道活性的显着丧失。水编辑 SSNMR 实验进一步确定,与野生型相比,TQ 突变体具有更大的整体溶剂暴露,但沿离子传导途径的水可及性降低,与通道的关闭状态一致。这些实验还表明,水在开放激活状态下靠近 KirBac1.1 的选择性过滤器,但它可能不会直接进入选择性过滤器。我们的研究结果表明,脂质结合启动了细胞质结构域亚基的协同旋转,这由多个亚基间盐桥稳定。这个动作使离子侧链远离大量水,同时允许水更多地进入 K+ 传导通路。这项工作突出了通用的膜蛋白基序,包括脂蛋白相互作用、结构域重排和水介导的扩散机制。































 京公网安备 11010802027423号
京公网安备 11010802027423号