当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reconstitution of the Ornithine Cycle with Arginine:Glycine Amidinotransferase to Engineer Escherichia coli into an Efficient Whole-Cell Catalyst of Guanidinoacetate.
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-07-23 , DOI: 10.1021/acssynbio.0c00138 Yiwen Zhang 1, 2, 3 , Hang Zhou 1, 2 , Yong Tao 1, 2 , Baixue Lin 1, 2
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-07-23 , DOI: 10.1021/acssynbio.0c00138 Yiwen Zhang 1, 2, 3 , Hang Zhou 1, 2 , Yong Tao 1, 2 , Baixue Lin 1, 2
Affiliation
|
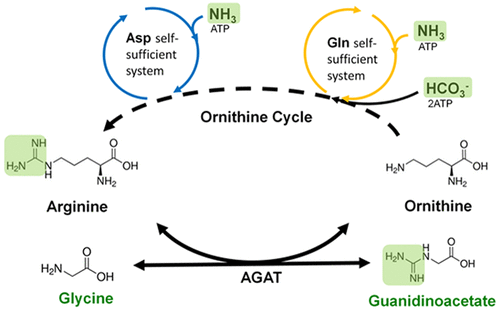
Guanidino compounds can be synthesized by transamidination reactions using arginine as a guanidine group donor. The efficiency of guanidino biosynthesis is often affected by the supply of arginine and the inhibition of the coproduct ornithine. To alleviate this shortcoming, we designed a reconstituted ornithine cycle in Escherichia coli to engineer an efficient whole-cell catalyst for guanidinoacetate (GAA) production by introducing a heterogeneous arginine:glycine amidinotransferase (AGAT). To alleviate the inhibition of ornithine, a citrulline synthetic module was constructed and optimized by introducing a glutamine self-sufficient system. Then, to improve the pathway from citrulline to arginine, an aspartate self-sufficient system was introduced into the arginine synthetic module. By combining these modules (GAA, citrulline, and arginine synthetic modules), a reconstituted ornithine cycle was developed, which significantly improved the biocatalyst efficiency (3.9-fold increase). In the system, arginine was regenerated efficiently through the reconstituted ornithine cycle, which converted arginine from a substrate to a cofactor for the transamidination reaction, thereby relieving the ornithine inhibition. Moreover, the amidino group of GAA in this system was mainly supplied by carbon and nitrogen assimilation. After the engineering process, 8.61 g/L GAA (73.56 mM) with a productivity of 0.39 g/L/h was achieved in a 22 h bioconversion. To the best of our knowledge, this is the first time that GAA has been produced in E. coli. This reconstructed ornithine cycle could be used as a transamidination platform for amidino group supply and has potential applications in the biosynthesis of other guanidino compounds.
中文翻译:

用精氨酸:甘氨酸酰胺基转移酶将鸟氨酸循环重建为工程大肠杆菌成为高效的胍基乙酸全细胞催化剂。
可以通过使用精氨酸作为胍基供体的氨基转移反应来合成胍基化合物。胍基生物合成的效率通常受精氨酸的供应和副产物鸟氨酸的抑制的影响。为了缓解这一缺点,我们设计了大肠杆菌中的鸟氨酸循环通过引入异质精氨酸:甘氨酸a基转移酶(AGAT),设计出一种高效的全细胞催化剂来生产胍基乙酸盐(GAA)。为了减轻鸟氨酸的抑制作用,通过引入谷氨酰胺自给系统构建并优化了瓜氨酸合成模块。然后,为了改善从瓜氨酸到精氨酸的途径,将天冬氨酸自给系统引入精氨酸合成模块中。通过组合这些模块(GAA,瓜氨酸和精氨酸合成模块),开发了重构的鸟氨酸循环,该循环显着提高了生物催化剂效率(提高了3.9倍)。在该系统中,精氨酸通过重组的鸟氨酸循环得以高效再生,从而将精氨酸从底物转化为辅助因子进行酰胺化反应,从而减轻了鸟氨酸的抑制作用。此外,该系统中GAA的a基主要由碳和氮同化作用提供。经过工程处理后,在22小时的生物转化中获得了8.61 g / L的GAA(73.56 mM),生产力为0.39 g / L / h。据我们所知,这是GAA首次在大肠杆菌。该重建的鸟氨酸循环可用作酰胺基团供应的转酰胺基化平台,并在其他胍基化合物的生物合成中具有潜在的应用。
更新日期:2020-08-21
中文翻译:

用精氨酸:甘氨酸酰胺基转移酶将鸟氨酸循环重建为工程大肠杆菌成为高效的胍基乙酸全细胞催化剂。
可以通过使用精氨酸作为胍基供体的氨基转移反应来合成胍基化合物。胍基生物合成的效率通常受精氨酸的供应和副产物鸟氨酸的抑制的影响。为了缓解这一缺点,我们设计了大肠杆菌中的鸟氨酸循环通过引入异质精氨酸:甘氨酸a基转移酶(AGAT),设计出一种高效的全细胞催化剂来生产胍基乙酸盐(GAA)。为了减轻鸟氨酸的抑制作用,通过引入谷氨酰胺自给系统构建并优化了瓜氨酸合成模块。然后,为了改善从瓜氨酸到精氨酸的途径,将天冬氨酸自给系统引入精氨酸合成模块中。通过组合这些模块(GAA,瓜氨酸和精氨酸合成模块),开发了重构的鸟氨酸循环,该循环显着提高了生物催化剂效率(提高了3.9倍)。在该系统中,精氨酸通过重组的鸟氨酸循环得以高效再生,从而将精氨酸从底物转化为辅助因子进行酰胺化反应,从而减轻了鸟氨酸的抑制作用。此外,该系统中GAA的a基主要由碳和氮同化作用提供。经过工程处理后,在22小时的生物转化中获得了8.61 g / L的GAA(73.56 mM),生产力为0.39 g / L / h。据我们所知,这是GAA首次在大肠杆菌。该重建的鸟氨酸循环可用作酰胺基团供应的转酰胺基化平台,并在其他胍基化合物的生物合成中具有潜在的应用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号