Nano Energy ( IF 16.8 ) Pub Date : 2020-07-24 , DOI: 10.1016/j.nanoen.2020.105128 Feiteng Wang , Yipeng Zhou , Sen Lin , Lijun Yang , Zheng Hu , Daiqian Xie
|
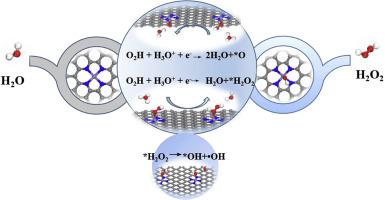
Iron and nitrogen co-doped carbons (Fe–N–C) have comparable activity to Pt-based catalysts for oxygen reduction reaction (ORR), but with much poorer durability in acidic electrolytes. Recently, regulating the coordination environment of Fe center (in-plane or axially) to boost the ORR activity of Fe–N–C has attracted many interests, and the axial OH ligand is even regarded as a necessary part of a highly-active structure. However, the influence of these regulations on the stability is still not clear. Herein, we performed kinetic and thermodynamic calculations based on density functional theory with explicit consideration of electrode potential to study the OH axial ligand effect on the stability of Fe–N–C electrocatalysts. We found that although the OH ligand can enhance the ORR onset potential to some extent, it substantially increases the H2O2 selectivity, pushing ORR diverted to the 2e- + 2e-pathway. In the latter 2e-process (H2O2 reduction), harmful hydroxyl radicals could be produced upon H2O2 dissociation. Therefore, from the perspective of catalysts’ stability, OH ligand coordination on the metal center is not a good way to develop stable ORR catalysts.
中文翻译:

轴向配体对酸性氧还原反应中Fe–N–C电催化剂稳定性的影响
铁和氮共掺杂碳(Fe–N–C)具有与基于Pt的氧还原反应(ORR)催化剂相当的活性,但在酸性电解液中的耐久性较差。最近,调节铁中心(平面或轴向)的配位环境以提高Fe–N–C的ORR活性引起了很多兴趣,甚至轴向OH配体甚至被认为是高活性结构的必要部分。但是,这些法规对稳定性的影响仍然不清楚。在这里,我们基于密度泛函理论进行了动力学和热力学计算,明确考虑了电极电势,以研究OH轴向配体对Fe–N–C电催化剂稳定性的影响。我们发现,尽管OH配体可以在一定程度上增强ORR发作的潜能,但它实际上会增加2 O 2选择性,推动ORR转向2e- + 2e途径。在后一种2e方法(H 2 O 2还原)中,H 2 O 2离解会产生有害的羟基自由基。因此,从催化剂的稳定性角度来看,金属中心的OH配位体配位不是开发稳定的ORR催化剂的好方法。






























 京公网安备 11010802027423号
京公网安备 11010802027423号