Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-07-24 , DOI: 10.1016/j.bioorg.2020.104136 Guanqun Zhan 1 , Rongkun Miao 1 , Fuxin Zhang 1 , Gang Chang 2 , Lei Zhang 1 , Xinxin Zhang 1 , Hui Zhang 1 , Zengjun Guo 1
|
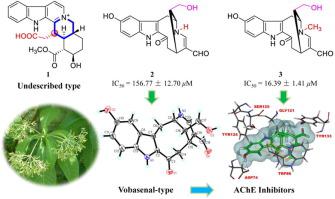
Seventeen monoterpene indole alkaloids, including seven new alkaloids (1–7) and ten known analogues (8–17), were isolated and identified from the leaves of R. vomitoria. The structures of new alkaloids were elucidated by extensive spectroscopic analysis and single-crystal X-ray diffraction analysis. Rauvomitorine I (1) represents the first example of an unprecedented C22 yohimbine-type monoterpene indole alkaloid featuring a carboxymethyl at C-14. The exceedingly rare vobasenal (2–3) and affinisine oxindole (5–6) framework type alkaloids are first reported from the Rauvolfia genus. Most notably, the structure of vobasenal-type alkaloids (2–3) were first determined by single-crystal X-ray diffraction analyses. Alkaloids 1–17 were tested their cytotoxicity against five cancer cell lines, however, none of them showed significant cytotoxicity at a concentration of 40 μM. All the isolated alkaloids were evaluated their acetylcholinesterase (AChE) inhibitory activities. Alkaloid 3 exhibited significant anti-AChE activity with an IC50 value of 16.39 ± 1.41 μM and alkaloids 8 and 10 showed moderate anti-AChE activities whereas the others (2, 9, 13, and 17) were weak inhibitors. This is the first report of vobasenal-type alkaloids as AChE inhibitors, indicating this type of alkaloids may be important sources for the discovery of new AChE inhibitors. A preliminary structure–activity relationship for AChE inhibitory activities showed the presence of the N-methyl group in vobasenal-type alkaloids may be essential for anti-AChE activity. Further molecular docking studies of vobasenal-type alkaloids revealed that interaction with Trp133 and Trp86 residues at hydrophobic subsite are necessary for the AChE inhibitory activities. This study not only enriches the chemical diversity of alkaloids in Apocynaceae plants but also provides new potential leading compounds and versatile scaffolds for the design and development of new AChE inhibitors to treat AD.
中文翻译:

紫杉叶片中具有乙酰胆碱酯酶抑制活性的单萜吲哚生物碱。
十七单萜吲哚生物碱,包括7种新生物碱(17)和10个的已知类似物(8 - 17),分离并从叶子识别R.萝芙木。广泛的光谱分析和单晶X射线衍射分析阐明了新生物碱的结构。Rauvomitorine I(1)代表了史无前例的C 22育亨宾型单萜吲哚生物碱的第一个实例,其特征在于C-14处的羧甲基。在极为罕见的vobasenal(2 - 3)和羟吲哚affinisine(5 - 6)框架型生物碱最早是从Rauvolfia属中报道的。最值得注意的是,vobasenal型生物碱(结构2 - 3)通过单晶X射线衍射分析是首先确定。测试了生物碱1 – 17对五种癌细胞系的细胞毒性,但是在40μM的浓度下,它们均未显示出明显的细胞毒性。评价所有分离的生物碱的乙酰胆碱酯酶(AChE)抑制活性。生物碱3表现出显着的抗AChE活性,IC 50值为16.39±1.41μM,生物碱8和10表现出中等抗乙酰胆碱酯酶活性而其他(2,9,13,和17)是弱抑制剂。这是vobasenal型生物碱作为AChE抑制剂的第一份报告,表明这种生物碱可能是发现新的AChE抑制剂的重要来源。AChE抑制活性的初步结构-活性关系表明存在N碱基型生物碱中的-甲基可能对抗AChE活性至关重要。vobasenal型生物碱的进一步分子对接研究表明,在疏水亚位点与Trp133和Trp86残基的相互作用对于AChE抑制活性是必需的。这项研究不仅丰富了夹竹桃植物中生物碱的化学多样性,而且还为设计和开发用于治疗AD的新型AChE抑制剂提供了潜在的领先化合物和多功能支架。































 京公网安备 11010802027423号
京公网安备 11010802027423号