当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Alkynylated and Dendronized 5-Aza-7-deazaguanine Nucleosides: Cross-Coupling with Tripropargylamine and Linear Alkynes, Click Functionalization, and Fluorescence of Pyrene Adducts†.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-07-23 , DOI: 10.1021/acs.joc.0c00926 Dasharath Kondhare 1 , Aigui Zhang 1 , Peter Leonard 1 , Frank Seela 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-07-23 , DOI: 10.1021/acs.joc.0c00926 Dasharath Kondhare 1 , Aigui Zhang 1 , Peter Leonard 1 , Frank Seela 1, 2
Affiliation
|
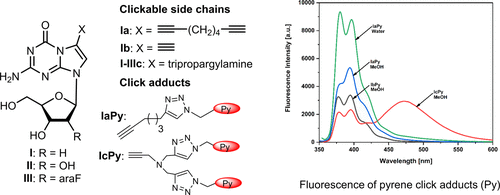
The change of the recognition face of 5-aza-7-deazaguanine bridgehead nucleosides with respect to purine nucleosides permits the construction of new purine–purine or purine–pyrimidine base pairs in DNA and RNA. Clickable derivatives of 5-aza-7-deazaguanine were synthesized by introducing ethynyl, 1,7-octadiynyl, and tripropargylamino side chains in the 7-position of the 5-aza-7-deazapurine moiety by Sonogashira cross-coupling. Click reactions were performed with 1-azidomethylpyrene by the copper-catalyzed azide–alkyne cycloaddition. The copper(I)-catalyzed click reaction on the tripropargylamino nucleoside was significantly faster and higher yielding than that for nucleosides carrying linear alkynyl chains. Also, this reaction could be performed with copper(II) as the catalyst. An autocatalyzed cycle was suggested in which the click product acts as a catalyst. Pyrene click adducts of linear alkynylated nucleosides showed pyrene monomer emission, while tripropargylamino adducts showed monomer and excimer fluorescence. The fluorescence intensities of the 5-aza-7-deazaguanine nucleosides were higher than those of their 7-deazaguanine counterparts. The reported clickable nucleosides can be utilized to functionalize or to cross-link monomeric nucleosides or DNA for diagnostic or imaging purposes and other applications in nucleic acid chemistry and biotechnology.
中文翻译:

炔化和Dendronized 5-Aza-7-脱氮鸟嘌呤核苷:与三炔丙基胺和线性炔烃的交叉偶联,点击官能化和P加合物的荧光作用†。
5-氮杂-7-脱氮鸟嘌呤桥头核苷相对于嘌呤核苷的识别面的改变允许在DNA和RNA中构建新的嘌呤-嘌呤或嘌呤-嘧啶碱基对。5-氮杂7-脱氮鸟嘌呤的可点击的衍生物是通过在由5-氮杂7-脱氮嘌呤部分的7位上引入乙炔基,1,7- octadiynyl,和tripropargylamino侧链合成的Sonogashira交叉耦合。通过铜催化的叠氮化物-炔烃环加成反应,使用1-叠氮甲基py进行点击反应。与带有线性炔基链的核苷相比,在三炔丙基氨基核苷上的铜(I)催化的点击反应明显更快且产率更高。同样,该反应可以用铜(II)作为催化剂进行。提出了一种自动催化的循环,其中点击产物充当催化剂。直链烷基化核苷的click点击加合物显示monomer单体发射,而三炔丙基氨基加合物显示单体和准分子荧光。5-aza-7-脱氮鸟嘌呤核苷的荧光强度高于其7-脱氮鸟嘌呤对应物的荧光强度。
更新日期:2020-08-21
中文翻译:

炔化和Dendronized 5-Aza-7-脱氮鸟嘌呤核苷:与三炔丙基胺和线性炔烃的交叉偶联,点击官能化和P加合物的荧光作用†。
5-氮杂-7-脱氮鸟嘌呤桥头核苷相对于嘌呤核苷的识别面的改变允许在DNA和RNA中构建新的嘌呤-嘌呤或嘌呤-嘧啶碱基对。5-氮杂7-脱氮鸟嘌呤的可点击的衍生物是通过在由5-氮杂7-脱氮嘌呤部分的7位上引入乙炔基,1,7- octadiynyl,和tripropargylamino侧链合成的Sonogashira交叉耦合。通过铜催化的叠氮化物-炔烃环加成反应,使用1-叠氮甲基py进行点击反应。与带有线性炔基链的核苷相比,在三炔丙基氨基核苷上的铜(I)催化的点击反应明显更快且产率更高。同样,该反应可以用铜(II)作为催化剂进行。提出了一种自动催化的循环,其中点击产物充当催化剂。直链烷基化核苷的click点击加合物显示monomer单体发射,而三炔丙基氨基加合物显示单体和准分子荧光。5-aza-7-脱氮鸟嘌呤核苷的荧光强度高于其7-脱氮鸟嘌呤对应物的荧光强度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号