当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical reinvestigation of the ozonolysis mechanism of allyl alcohol
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-07-23 , DOI: 10.1002/qua.26387 Xiaoyang Lei 1 , Weina Wang 1 , Wenliang Wang 1
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-07-23 , DOI: 10.1002/qua.26387 Xiaoyang Lei 1 , Weina Wang 1 , Wenliang Wang 1
Affiliation
|
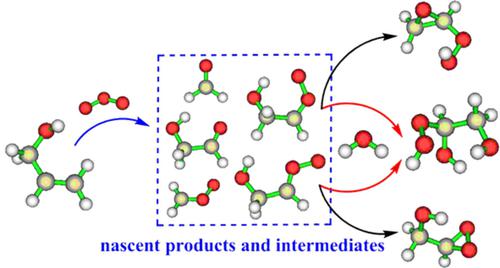
Allyl alcohol (AA) is the simplest unsaturated alcohol. Ozonolysis is one of the key removal processes for AA in the atmosphere. However, a recent theoretical study suggests that the ozonolysis of AA cannot feasibly occur in atmospheric conditions because of the high barrier (~96 kcal/mol) involved in the primary ozonide (POZ) decomposition. In this work, the ozonolysis mechanism of AA was reinvestigated theoretically. The computed barrier for POZ decomposition is only ~20 kcal/mol. Therefore, the AA ozonolysis can take place in the atmosphere, consistent with the experimental conclusions. Moreover, two new Criegee intermediates (syn‐ and anti‐AA‐CI) were found to be produced in this reaction. The wave function analyses indicate that there exists an intermolecular hydrogen bond in syn‐AA‐CI, which significantly affects its unimolecular decomposition and reactions with H2O. Compared with the normal reactions of anti‐CI‐AA, the stabilized syn‐AA‐CI has two distinct isomerization channels: (i) addition of OH group to the reactive sites of CI forming an ethylene oxide (HOOCH2OCH2) and (ii) double H‐transfer producing HOOCH2CHO. Meanwhile, the addition of H2O in syn‐AA‐CI also exhibits two different pathways. One is the unique addition‐coupled double H‐transfer, and the other is the addition‐coupled single H‐transfer, both leading to the formation of CH2(OH)CH(OH)OOH.
中文翻译:

理论研究烯丙醇的臭氧分解机理
烯丙醇(AA)是最简单的不饱和醇。臭氧分解是去除大气中AA的关键过程之一。但是,最近的一项理论研究表明,由于在主要的臭氧层(POZ)分解中涉及的高势垒(〜96 kcal / mol),AA的臭氧分解不能在大气条件下发生。在这项工作中,从理论上重新研究了AA的臭氧分解机理。计算出的POZ分解势垒仅为〜20 kcal / mol。因此,与实验结论一致,AA臭氧分解可以在大气中进行。此外,在该反应中发现了两种新的Criegee中间体(syn-和anti -AA-CI)。波函数分析表明,分子间存在分子间氢键。syn -AA -CI,显着影响其单分子分解和与H 2 O的反应。与抗CI -AA的正常反应相比,稳定的syn -AA -CI具有两个不同的异构化通道:(i)添加OH基团与CI的反应部位形成环氧乙烷(HOOCH 2 OCH 2)和(ii)产生H转移的双HOOCH 2 CHO。同时,在syn -AA-CI中添加H 2 O也表现出两种不同的途径。一种是独特的加成偶联双H转移,另一种是加成偶联单H转移,两者均导致形成CH 2(OH)CH(OH)OOH。
更新日期:2020-07-23
中文翻译:

理论研究烯丙醇的臭氧分解机理
烯丙醇(AA)是最简单的不饱和醇。臭氧分解是去除大气中AA的关键过程之一。但是,最近的一项理论研究表明,由于在主要的臭氧层(POZ)分解中涉及的高势垒(〜96 kcal / mol),AA的臭氧分解不能在大气条件下发生。在这项工作中,从理论上重新研究了AA的臭氧分解机理。计算出的POZ分解势垒仅为〜20 kcal / mol。因此,与实验结论一致,AA臭氧分解可以在大气中进行。此外,在该反应中发现了两种新的Criegee中间体(syn-和anti -AA-CI)。波函数分析表明,分子间存在分子间氢键。syn -AA -CI,显着影响其单分子分解和与H 2 O的反应。与抗CI -AA的正常反应相比,稳定的syn -AA -CI具有两个不同的异构化通道:(i)添加OH基团与CI的反应部位形成环氧乙烷(HOOCH 2 OCH 2)和(ii)产生H转移的双HOOCH 2 CHO。同时,在syn -AA-CI中添加H 2 O也表现出两种不同的途径。一种是独特的加成偶联双H转移,另一种是加成偶联单H转移,两者均导致形成CH 2(OH)CH(OH)OOH。

































 京公网安备 11010802027423号
京公网安备 11010802027423号