Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-07-23 , DOI: 10.1016/j.tetlet.2020.152280 Alex Boateng , Tokifumi Harada , Yasuhiko Ashikari , Makoto Nakajima , Masaharu Sugiura
|
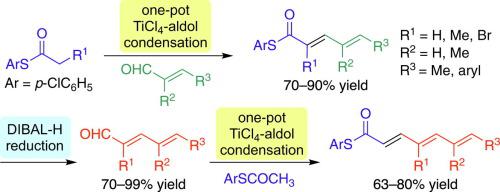
TiCl4-promoted aldol condensations of S-4-chlorophenyl thioesters with enals or dienals led to the production of dienyl or trienyl thioesters in good yields. Due to good crystallinity, products with high E/Z ratios were obtained by simple filtration in many cases. Reduction of the thioester moiety by DIBAL-H afforded the corresponding unsaturated aldehydes while suppressing the E/Z-isomerization and overreduction. The method was applied to the preparation of simple synthetic intermediates of natural products such as spectinabilin and aureothin. A bromotrienyl thioester product was converted to a tetraenyl thioester via a palladium-catalyzed cross-coupling without affecting the thioester moiety.
中文翻译:

共轭多不硫酯的合成通过一锅煮的TiCl 4促进的醛醇缩合
TiCl 4促进的S -4-氯苯基硫酯与烯醛或二烯醛的醛醇缩合导致二烯基或三烯基硫酯的高收率生产。由于良好的结晶度,在许多情况下,通过简单的过滤即可获得具有高E / Z比的产品。用DIBAL-H还原硫酯部分可得到相应的不饱和醛,同时抑制E / Z异构化和过度还原。该方法可用于制备天然产物如鬼臼菌素和金黄色素的简单合成中间体。溴三烯基硫酯产物通过以下方式转化为四烯基硫酯 钯催化的交叉偶联而不会影响硫酯部分。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号