Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-07-23 , DOI: 10.1016/j.bioorg.2020.104130 Chhabi Lal Chaudhary 1 , Prakash Chaudhary 1 , Sadan Dahal 1 , Dawon Bae 1 , Tae-Gyu Nam 2 , Jung-Ae Kim 1 , Byeong-Seon Jeong 1
|
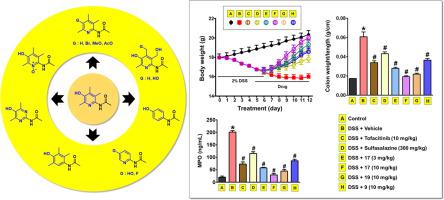
6-Aminopyridin-3-ol scaffold has shown an excellent anti-inflammatory bowel disease activity. Various analogues with the scaffold were synthesized in pursuit of the diversity of side chains tethering on the C(6)-position. Structure-activity relationship among the analogues was investigated to understand the effects of the side chains and their linkers on their anti-inflammatory activities. In this study, structural modification moved beyond side chains on the C(6)-position and reached to pyridine ring itself. It expedited us to synthesize diverse ring-modified analogues of a representative pyridine-3-ol, 6-acetamido-2,4,5-trimethylpyridin-3-ol (9). In the evaluation of compounds on their inhibitory actions against TNF-α-induced adhesion of monocytic cells to colonic epithelial cells, an in vitro model mimicking colon inflammation, the effects of compounds 9, 17, and 19 were greater than tofacitinib, an orally available anti-colitis drug, and compound 17 showed the greatest activity. In addition, TNF-α-induced angiogenesis, which permits more inflammatory cell migration into inflamed tissues, was significantly blocked by compounds 17 and 19 in a concentration-dependent manner. In the comparison of in vivo therapeutic effects of compounds 9, 17, and 19 on dextran sulfate sodium (DSS)-induced colitis in mice, compound 17 was the most potent and efficacious, and compound 19 was better than compound 9 which showed a similar degree of inhibitory effect to tofacitinib. Taken together, it seems that either the trimethyl system or the hydroxyl group on the pyridinol ring is essential to the activity. This finding might become a new milestone in the development of pyridinol-based anti-inflammatory bowel disease agents.
中文翻译:

通过6-乙酰氨基-2,4,5-三甲基吡啶-3-醇的环修饰类似物抑制结肠炎。
6-Aminopyridin-3-ol支架具有出色的抗炎肠病活性。合成与支架的各种类似物,以追求束缚在C(6)位置的侧链的多样性。研究了类似物之间的构效关系,以了解侧链及其接头对抗炎活性的影响。在这项研究中,结构修饰移动到C(6)-位上的侧链之外,并到达吡啶环本身。它加速了我们合成代表性的吡啶-3-醇,6-乙酰氨基-2,4,5-三甲基吡啶-3-醇的各种环修饰类似物(9)。在他们的血管紧张素Ⅱ受体拮抗作用的化合物的评估TNF-α诱导的单核细胞的粘附到结肠上皮细胞,在体外模型模拟结肠炎,化合物的作用9,17,和19比托法替尼是更大的,可口服抗结肠炎药和化合物17表现出最大的活性。此外,化合物17和19以浓度依赖的方式显着阻断了TNF-α诱导的血管生成,该血管生成允许更多的炎症细胞迁移到发炎的组织中。在的比较在体内的化合物的治疗效果9,17,以及针对硫酸葡聚糖硫酸钠(DSS)诱导的结肠炎中的19,化合物17最有效,最有效,化合物19优于化合物9,后者的抑制作用与托法替尼相似。两者合计,似乎三甲基系统或吡啶环上的羟基对于该活性是必不可少的。这一发现可能成为基于吡啶醇的消炎性肠病药物开发的新里程碑。











































 京公网安备 11010802027423号
京公网安备 11010802027423号