Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Studies of Dynamic Binding of Amino Acids to TiO2 Nanoparticle Surfaces by Solution NMR and Molecular Dynamics Simulations.
Langmuir ( IF 3.7 ) Pub Date : 2020-07-21 , DOI: 10.1021/acs.langmuir.0c01256 Mengjun Xue 1 , Janani Sampath 2 , Rachel N Gebhart 1 , Havard J Haugen 3 , S Petter Lyngstadaas 3 , Jim Pfaendtner 2 , Gary Drobny 1
Langmuir ( IF 3.7 ) Pub Date : 2020-07-21 , DOI: 10.1021/acs.langmuir.0c01256 Mengjun Xue 1 , Janani Sampath 2 , Rachel N Gebhart 1 , Havard J Haugen 3 , S Petter Lyngstadaas 3 , Jim Pfaendtner 2 , Gary Drobny 1
Affiliation
|
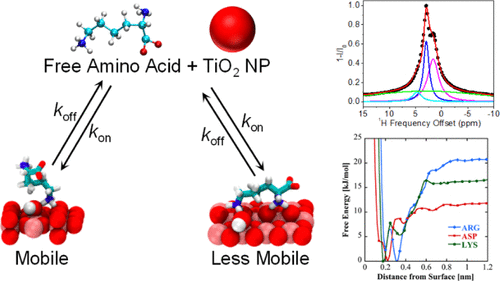
Adsorption of biomolecules onto material surfaces involves a potentially complex mechanism where molecular species interact to varying degrees with a heterogeneous material surface. Surface adsorption studies by atomic force microscopy, sum frequency generation spectroscopy, and solid-state NMR detect the structures and interactions of biomolecular species that are bound to material surfaces, which, in the absence of a solid–liquid interface, do not exchange rapidly between surface-bound forms and free molecular species in bulk solution. Solution NMR has the potential to complement these techniques by detecting and studying transiently bound biomolecules at the liquid–solid interface. Herein, we show that dark-state exchange saturation transfer (DEST) NMR experiments on gel-stabilized TiO2 nanoparticle (NP) samples detect several forms of biomolecular adsorption onto titanium(IV) oxide surfaces. Specifically, we use the DEST approach to study the interaction of amino acids arginine (Arg), lysine (Lys), leucine (Leu), alanine (Ala), and aspartic acid (Asp) with TiO2 rutile NP surfaces. Whereas Leu, Ala, and Asp display only a single weakly interacting form in the presence of TiO2 NPs, Arg and Lys displayed at least two distinct bound forms: a species that is surface bound and retains a degree of reorientational motion and a second more tightly bound form characterized by broadened DEST profiles upon the addition of TiO2 NPs. Molecular dynamics simulations indicate different surface bound states for both Lys and Arg depending on the degree of TiO2 surface hydroxylation but only a single bound state for Asp regardless of the degree of surface hydroxylation, in agreement with results obtained from the analysis of DEST profiles.
中文翻译:

通过溶液核磁共振和分子动力学模拟研究氨基酸与二氧化钛纳米颗粒表面的动态结合。
生物分子吸附到材料表面涉及一种潜在的复杂机制,其中分子种类与异质材料表面发生不同程度的相互作用。通过原子力显微镜、和频发生光谱和固态 NMR 进行的表面吸附研究检测了与材料表面结合的生物分子物质的结构和相互作用,在没有固液界面的情况下,它们之间不会快速交换本体溶液中的表面结合形式和自由分子种类。溶液 NMR 有可能通过检测和研究液固界面处的瞬时结合生物分子来补充这些技术。在此,我们展示了凝胶稳定的 TiO 2的暗态交换饱和转移 (DEST) NMR 实验纳米颗粒 (NP) 样品检测到氧化钛 (IV) 表面上的多种生物分子吸附形式。具体来说,我们使用 DEST 方法研究氨基酸精氨酸 (Arg)、赖氨酸 (Lys)、亮氨酸 (Leu)、丙氨酸 (Ala) 和天冬氨酸 (Asp) 与 TiO 2金红石 NP 表面的相互作用。虽然 Leu、Ala 和 Asp 在 TiO 2 NPs 存在下仅显示出单一的弱相互作用形式,但 Arg 和 Lys 显示出至少两种不同的结合形式:一种是表面结合并保持一定程度的重新定向运动,另一种是更多紧密结合的形式,特征在于添加 TiO 2后 DEST 谱变宽NP。分子动力学模拟表明 Lys 和 Arg 的不同表面结合态取决于 TiO 2表面羟基化的程度,但无论表面羟基化程度如何,Asp 都只有单一结合态,这与从 DEST 曲线分析获得的结果一致。
更新日期:2020-09-09
中文翻译:

通过溶液核磁共振和分子动力学模拟研究氨基酸与二氧化钛纳米颗粒表面的动态结合。
生物分子吸附到材料表面涉及一种潜在的复杂机制,其中分子种类与异质材料表面发生不同程度的相互作用。通过原子力显微镜、和频发生光谱和固态 NMR 进行的表面吸附研究检测了与材料表面结合的生物分子物质的结构和相互作用,在没有固液界面的情况下,它们之间不会快速交换本体溶液中的表面结合形式和自由分子种类。溶液 NMR 有可能通过检测和研究液固界面处的瞬时结合生物分子来补充这些技术。在此,我们展示了凝胶稳定的 TiO 2的暗态交换饱和转移 (DEST) NMR 实验纳米颗粒 (NP) 样品检测到氧化钛 (IV) 表面上的多种生物分子吸附形式。具体来说,我们使用 DEST 方法研究氨基酸精氨酸 (Arg)、赖氨酸 (Lys)、亮氨酸 (Leu)、丙氨酸 (Ala) 和天冬氨酸 (Asp) 与 TiO 2金红石 NP 表面的相互作用。虽然 Leu、Ala 和 Asp 在 TiO 2 NPs 存在下仅显示出单一的弱相互作用形式,但 Arg 和 Lys 显示出至少两种不同的结合形式:一种是表面结合并保持一定程度的重新定向运动,另一种是更多紧密结合的形式,特征在于添加 TiO 2后 DEST 谱变宽NP。分子动力学模拟表明 Lys 和 Arg 的不同表面结合态取决于 TiO 2表面羟基化的程度,但无论表面羟基化程度如何,Asp 都只有单一结合态,这与从 DEST 曲线分析获得的结果一致。































 京公网安备 11010802027423号
京公网安备 11010802027423号