当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
4-Fluorobenzylpiperazine-Containing Derivatives as Efficient Inhibitors of Mushroom Tyrosinase.
ChemMedChem ( IF 3.6 ) Pub Date : 2020-07-21 , DOI: 10.1002/cmdc.202000125 Serena Vittorio 1 , Laura Ielo 2 , Salvatore Mirabile 1 , Rosaria Gitto 1 , Antonella Fais 3 , Sonia Floris 3 , Antonio Rapisarda 1 , Maria Paola Germanò 1 , Laura De Luca 1
ChemMedChem ( IF 3.6 ) Pub Date : 2020-07-21 , DOI: 10.1002/cmdc.202000125 Serena Vittorio 1 , Laura Ielo 2 , Salvatore Mirabile 1 , Rosaria Gitto 1 , Antonella Fais 3 , Sonia Floris 3 , Antonio Rapisarda 1 , Maria Paola Germanò 1 , Laura De Luca 1
Affiliation
|
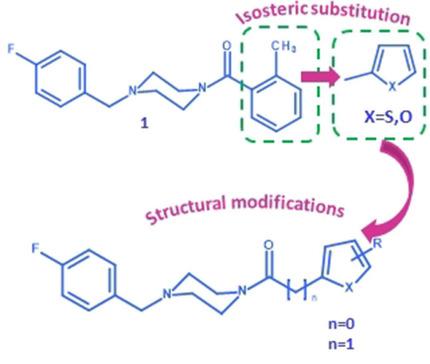
Tyrosinase is a type‐3 copper protein involved in the biosynthesis of melanin pigments; therefore, the inhibition of its enzymatic activity represents a promising strategy for the treatment of hyperpigmentation‐related disorders. To address this point, we previously designed a class of 4‐(4‐fluorobenzyl)piperazin‐1‐yl‐based compounds, which proved to be more active inhibitors against tyrosinase from mushroom Agaricus bisporus than the positive control kojic acid. Herein, we report the synthesis of further series of 4‐(4‐fluorobenzyl)piperazin‐1‐yl analogues bearing a (hetero)aromatic fragment as key feature to improve protein affinity. The newly synthesized compounds were assayed in vitro and proved to be potent inhibitors in the low‐micromolar range. The active 2‐thienyl and 2‐furyl derivatives were selected for further modification to allow their binding mode to be analyzed by docking studies and to give satisfactory safety profiles.
中文翻译:

含 4-氟苄基哌嗪的衍生物作为蘑菇酪氨酸酶的有效抑制剂。
酪氨酸酶是一种参与黑色素生物合成的 3 型铜蛋白;因此,抑制其酶活性是治疗色素沉着相关疾病的一种有前景的策略。为了解决这一点,我们之前设计了一类基于 4-(4-氟苄基)哌嗪-1-基的化合物,与阳性对照曲酸相比,它被证明是更有效的双孢蘑菇酪氨酸酶抑制剂。在此,我们报告了进一步系列的 4-(4-氟苄基)哌嗪-1-基类似物的合成,其带有(杂)芳族片段作为提高蛋白质亲和力的关键特征。新合成的化合物在体外进行了分析并被证明是低微摩尔范围内的有效抑制剂。选择活性 2-噻吩基和 2-呋喃基衍生物进行进一步修饰,以允许通过对接研究分析它们的结合模式并提供令人满意的安全性。
更新日期:2020-09-14
中文翻译:

含 4-氟苄基哌嗪的衍生物作为蘑菇酪氨酸酶的有效抑制剂。
酪氨酸酶是一种参与黑色素生物合成的 3 型铜蛋白;因此,抑制其酶活性是治疗色素沉着相关疾病的一种有前景的策略。为了解决这一点,我们之前设计了一类基于 4-(4-氟苄基)哌嗪-1-基的化合物,与阳性对照曲酸相比,它被证明是更有效的双孢蘑菇酪氨酸酶抑制剂。在此,我们报告了进一步系列的 4-(4-氟苄基)哌嗪-1-基类似物的合成,其带有(杂)芳族片段作为提高蛋白质亲和力的关键特征。新合成的化合物在体外进行了分析并被证明是低微摩尔范围内的有效抑制剂。选择活性 2-噻吩基和 2-呋喃基衍生物进行进一步修饰,以允许通过对接研究分析它们的结合模式并提供令人满意的安全性。















































 京公网安备 11010802027423号
京公网安备 11010802027423号