当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Enolate-Structure-Enabled Anionic Cascade Cyclization Reaction: Easy Access to Complex Scaffolds with Contiguous Six-, Five-, and Four-Membered Rings.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-22 , DOI: 10.1002/anie.202008317
Tomas Javorskis 1, 2 , Ieva Karpavičienė 1 , Arminas Jurys 1 , Gustautas Snarskis 2 , Rita Bukšnaitienė 1 , Edvinas Orentas 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-22 , DOI: 10.1002/anie.202008317
Tomas Javorskis 1, 2 , Ieva Karpavičienė 1 , Arminas Jurys 1 , Gustautas Snarskis 2 , Rita Bukšnaitienė 1 , Edvinas Orentas 1
Affiliation
|
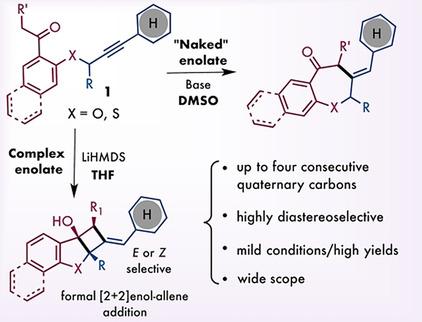
Catalyst‐free addition of ketone enolate to non‐activated multiple C−C bonds involves non‐complementary reaction partners and typically requires super‐basic conditions. On the other hand, highly aggregated or solvated enolates are not reactive enough to undergo direct addition to alkenes or alkynes. Herein, we report a new anionic cascade reaction for one‐step assembly of intriguing molecular scaffolds possessing contiguous six‐, five‐, and four‐membered rings, representing a formal [2+2] enol–allene cycloaddition. Reaction proceeds under very mild conditions and with excellent diastereoselectivity. Deeper mechanistic and computational studies revealed unusually slow proton transfer phenomenon in cyclic ketone intermediate and explained peculiar stereochemical outcome.
中文翻译:

能够启用Enolate结构的阴离子级联环化反应:轻松访问具有连续六元,五元和四元环的复杂支架。
在非活化的多个C-C键上无催化剂地添加烯醇酮涉及非互补反应伙伴,通常需要超碱性条件。另一方面,高度聚集或溶剂化的烯酸酯的反应性不足以直接加成到烯烃或炔烃中。本文中,我们报道了一个新的阴离子级联反应,该步骤用于一步步组装具有连续六元,五元和四元环的有趣分子支架,代表正式的[2 + 2]烯醇-丙二烯环加成反应。反应在非常温和的条件下进行,并且具有非对映选择性。更深入的机械和计算研究表明,在环酮中间体中质子转移现象异常缓慢,并解释了特殊的立体化学结果。
更新日期:2020-07-22
中文翻译:

能够启用Enolate结构的阴离子级联环化反应:轻松访问具有连续六元,五元和四元环的复杂支架。
在非活化的多个C-C键上无催化剂地添加烯醇酮涉及非互补反应伙伴,通常需要超碱性条件。另一方面,高度聚集或溶剂化的烯酸酯的反应性不足以直接加成到烯烃或炔烃中。本文中,我们报道了一个新的阴离子级联反应,该步骤用于一步步组装具有连续六元,五元和四元环的有趣分子支架,代表正式的[2 + 2]烯醇-丙二烯环加成反应。反应在非常温和的条件下进行,并且具有非对映选择性。更深入的机械和计算研究表明,在环酮中间体中质子转移现象异常缓慢,并解释了特殊的立体化学结果。

































 京公网安备 11010802027423号
京公网安备 11010802027423号