当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 1-(tert-Butyl) 4-Methyl (1R,2S,4R)-2-Methylcyclohexane-1,4-dicarboxylate from Hagemann's tert-Butyl Ester for an Improved Synthesis of BMS-986251.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-07-20 , DOI: 10.1021/acs.joc.0c01169 Lyndon A M Cornelius 1 , Jianqing Li 1 , Daniel Smith 1 , Subramaniam Krishnananthan 1 , Shiuhang Yip 1 , Dauh-Rurng Wu 1 , Joseph Pawluczyk 1 , Darpandeep Aulakh 2 , Amy A Sarjeant 2 , James Kempson 1 , Joseph A Tino 1 , Arvind Mathur , T G Murali Dhar 1 , Robert J Cherney 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-07-20 , DOI: 10.1021/acs.joc.0c01169 Lyndon A M Cornelius 1 , Jianqing Li 1 , Daniel Smith 1 , Subramaniam Krishnananthan 1 , Shiuhang Yip 1 , Dauh-Rurng Wu 1 , Joseph Pawluczyk 1 , Darpandeep Aulakh 2 , Amy A Sarjeant 2 , James Kempson 1 , Joseph A Tino 1 , Arvind Mathur , T G Murali Dhar 1 , Robert J Cherney 1
Affiliation
|
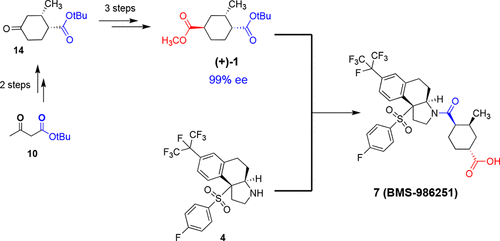
We describe an efficient synthetic route to differentially protected diester, 1-(tert-butyl) 4-methyl (1R,2S,4R)-2-methylcyclohexane-1,4-dicarboxylate (+)-1, via palladium-catalyzed methoxycarbonylation of an enol triflate derived from a Hagemann’s ester derivative followed by a stereoselective Crabtree hydrogenation. Diester 1 is a novel chiral synthon useful in drug discovery and was instrumental in the generation of useful SAR during a RORγt inverse agonist program. In addition, we describe a second-generation synthesis of the clinical candidate BMS-986251, using diester 1 as a critical component.
中文翻译:

由哈格曼叔丁基酯合成1-(叔丁基)4-甲基(1R,2S,4R)-2-甲基环己烷-1,4-二羧酸酯,用于改进BMS-986251的合成。
我们描述了一种有效的合成路线,可通过钯-来实现差动保护的二酯1-(叔丁基)4-甲基(1 R,2 S,4 R)-2-甲基环己烷-1,4-二羧酸(+)- 1催化由Hagemann酯衍生物衍生的烯醇三氟甲磺酸酯的甲氧基羰基化,然后进行立体选择性Crabtree氢化。Diester 1是一种新颖的手性合成子,可用于药物开发,并且在RORγt反向激动剂程序过程中有助于生成有用的SAR。此外,我们描述了使用二酯1作为关键成分的临床候选药物BMS-986251的第二代合成。
更新日期:2020-08-21
中文翻译:

由哈格曼叔丁基酯合成1-(叔丁基)4-甲基(1R,2S,4R)-2-甲基环己烷-1,4-二羧酸酯,用于改进BMS-986251的合成。
我们描述了一种有效的合成路线,可通过钯-来实现差动保护的二酯1-(叔丁基)4-甲基(1 R,2 S,4 R)-2-甲基环己烷-1,4-二羧酸(+)- 1催化由Hagemann酯衍生物衍生的烯醇三氟甲磺酸酯的甲氧基羰基化,然后进行立体选择性Crabtree氢化。Diester 1是一种新颖的手性合成子,可用于药物开发,并且在RORγt反向激动剂程序过程中有助于生成有用的SAR。此外,我们描述了使用二酯1作为关键成分的临床候选药物BMS-986251的第二代合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号