Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-07-21 , DOI: 10.1016/j.tetlet.2020.152253 Chunmei Li , Weiming Fan , Chenze Qi , Furen Zhang
|
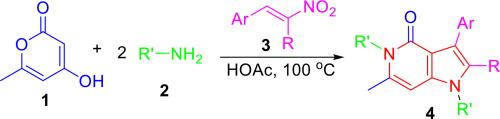
A direct and metal-free access toward poly-substituted pyrrolo[3,2-c]pyridin-4-one and its analogues have been established through a simple four-component, two-step reaction of 4-hydroxy-6-methyl-2H-pyran-2-one, two amine molecules, and β-nitrostyrenes. The reaction allows the formation of five different C–N, C–C bonds by the multiple bond cleavage only using acetic acid as catalyst and solvent. The advantages of broad substrate scope, efficient and metal-free reaction conditions, inexpensive and readily available starting materials as well as simple one-pot two-step operation, make the strategy highly attractive. This transformation in acetic acid resulted in the rapid establishment of functional bioactive poly-substituted pyrrolo[3,2-c]pyridin-4-one derivatives.
中文翻译:

吡咯并[3,2 - c ]吡啶-4-酮衍生物的四组分合成
通过简单的四组分,两步4-羟基-6-甲基-四氢呋喃反应,已经建立了直接,无金属的多取代吡咯并[3,2 - c ]吡啶-4-酮及其类似物的途径。 2 H-吡喃-2-一,两个胺分子和β-硝基苯乙烯。该反应仅通过使用乙酸作为催化剂和溶剂,即可通过多重键裂解形成五个不同的C–N,C–C键。广泛的底物范围,高效且无金属的反应条件,廉价且易于获得的起始原料以及简单的一锅两步操作等优点使该策略具有很高的吸引力。乙酸的这种转化导致功能性生物活性多取代吡咯并[3,2- c] pyridin-4-one衍生物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号