当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functional 4H-Dithieno[3,2-b:2′,3′-d]pyrrole Derivatives in Base-Dopable Conjugated Polymers and Oligomers
Macromolecules ( IF 5.1 ) Pub Date : 2020-07-20 , DOI: 10.1021/acs.macromol.0c01071
Joshua P. Green 1 , Haojie Dai 1 , Filip Aniés 1 , Martin Heeney 1
Macromolecules ( IF 5.1 ) Pub Date : 2020-07-20 , DOI: 10.1021/acs.macromol.0c01071
Joshua P. Green 1 , Haojie Dai 1 , Filip Aniés 1 , Martin Heeney 1
Affiliation
|
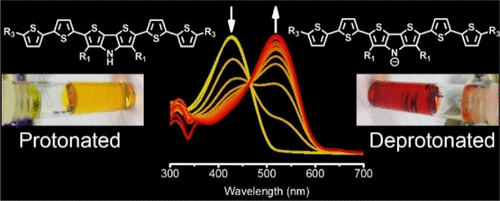
A novel derivative of dithieno[3,2-b:2′,3′-d]pyrrole containing solubilizing side chains in the β-positions is reported and incorporated into a conjugated oligomer by a Pd-catalyzed direct arylation reaction. Following deprotection, a soluble conjugated oligomer was formed, in which the pyrrolic nitrogen was unsubstituted. Deprotonation upon exposure to tetrabutylammonium fluoride or hydroxide resulted in a large shift of the main absorption peak of 0.53 eV, from 425 to 520 nm, as a result of the delocalization of the resulting anion. Such changes were easily observable by the naked eye, and the pKa value of the pyrrolic group was calculated to be ∼15.9. The dithieno[3,2-b:2′,3′-d]pyrrole unit was also incorporated into an analogous polymeric system, which was partly deprotected under the same conditions as that of the conjugated oligomer. The resulting polymer was readily deprotonated in solution, resulting in a shift of the main absorption peak by 0.55 eV.
中文翻译:

碱掺杂共轭聚合物和低聚物中的功能性4 H -Dithieno [3,2- b:2',3'- d ]吡咯衍生物
报道了在β-位含有可溶侧链的二噻吩并[3,2- b:2',3'- d ]吡咯的新型衍生物,并通过Pd催化的直接芳基化反应掺入到共轭低聚物中。脱保护后,形成可溶性共轭低聚物,其中吡咯氮未被取代。暴露于氟化四丁基铵或氢氧化物上的去质子化导致0.53 eV的主吸收峰从425 nm移至520 nm,这是所产生阴离子的离域作用的大偏移。用肉眼很容易观察到这种变化,并且吡咯基的p K a值经计算为〜15.9。dithieno [3,2- b:2',3'- d也将]吡咯单元并入类似的聚合物体系中,该体系在与共轭低聚物相同的条件下被部分脱保护。所得聚合物易于在溶液中去质子化,导致主吸收峰移动0.55 eV。
更新日期:2020-08-11
中文翻译:

碱掺杂共轭聚合物和低聚物中的功能性4 H -Dithieno [3,2- b:2',3'- d ]吡咯衍生物
报道了在β-位含有可溶侧链的二噻吩并[3,2- b:2',3'- d ]吡咯的新型衍生物,并通过Pd催化的直接芳基化反应掺入到共轭低聚物中。脱保护后,形成可溶性共轭低聚物,其中吡咯氮未被取代。暴露于氟化四丁基铵或氢氧化物上的去质子化导致0.53 eV的主吸收峰从425 nm移至520 nm,这是所产生阴离子的离域作用的大偏移。用肉眼很容易观察到这种变化,并且吡咯基的p K a值经计算为〜15.9。dithieno [3,2- b:2',3'- d也将]吡咯单元并入类似的聚合物体系中,该体系在与共轭低聚物相同的条件下被部分脱保护。所得聚合物易于在溶液中去质子化,导致主吸收峰移动0.55 eV。































 京公网安备 11010802027423号
京公网安备 11010802027423号