当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Hydroxycyclopropanol Ring-Opening Carbonylative Lactonization to Fused Bicyclic Lactones
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-20 , DOI: 10.1021/jacs.0c06179 Xinpei Cai 1 , Weida Liang 1 , Mingxin Liu 1 , Xiating Li 1 , Mingji Dai 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-20 , DOI: 10.1021/jacs.0c06179 Xinpei Cai 1 , Weida Liang 1 , Mingxin Liu 1 , Xiating Li 1 , Mingji Dai 1
Affiliation
|
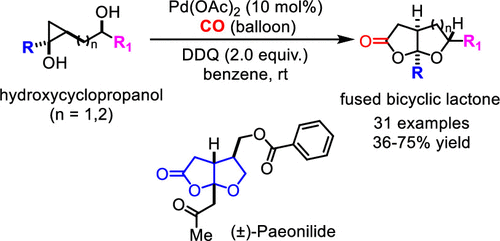
A novel palladium-catalyzed ring opening carbonylative lactonization of readily available hydroxycyclopropanols was developed to efficiently synthesize tetrahydrofuran (THF) or tetrahydropyran (THP)-fused bicyclic γ-lactones, two privileged scaffolds often found in natural products. The reaction features mild reaction conditions, good functional group tolerability, and scalability. Its application was demonstrated in a short total synthesis of (±)-paeonilide. The fused bicyclic γ-lactone products can be easily diversified to other medicinally important scaffolds, which further broadens the application of this new carbonylation method.
中文翻译:

催化羟基环丙醇开环羰基内酯化生成稠合双环内酯
开发了一种新型钯催化的易得羟基环丙醇的开环羰基内酯化反应,以有效合成四氢呋喃(THF)或四氢吡喃(THP)稠合双环γ-内酯,这是天然产物中常见的两种特殊支架。该反应具有反应条件温和、官能团耐受性好、可扩展等特点。它的应用在(±)-芍药内酯的简短全合成中得到了证明。稠合双环γ-内酯产品可以很容易地多样化为其他医学上重要的支架,这进一步拓宽了这种新的羰基化方法的应用。
更新日期:2020-07-20
中文翻译:

催化羟基环丙醇开环羰基内酯化生成稠合双环内酯
开发了一种新型钯催化的易得羟基环丙醇的开环羰基内酯化反应,以有效合成四氢呋喃(THF)或四氢吡喃(THP)稠合双环γ-内酯,这是天然产物中常见的两种特殊支架。该反应具有反应条件温和、官能团耐受性好、可扩展等特点。它的应用在(±)-芍药内酯的简短全合成中得到了证明。稠合双环γ-内酯产品可以很容易地多样化为其他医学上重要的支架,这进一步拓宽了这种新的羰基化方法的应用。































 京公网安备 11010802027423号
京公网安备 11010802027423号