当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel‐Catalyzed Synthesis of N‐(Hetero)aryl Carbamates from Cyanate Salts and Phenols Activated with Cyanuric Chloride
ChemCatChem ( IF 3.8 ) Pub Date : 2020-07-19 , DOI: 10.1002/cctc.202000876
Iman Dindarloo Inaloo 1 , Mohsen Esmaeilpour 1, 2 , Sahar Majnooni 3 , Ali Reza Oveisi 4
ChemCatChem ( IF 3.8 ) Pub Date : 2020-07-19 , DOI: 10.1002/cctc.202000876
Iman Dindarloo Inaloo 1 , Mohsen Esmaeilpour 1, 2 , Sahar Majnooni 3 , Ali Reza Oveisi 4
Affiliation
|
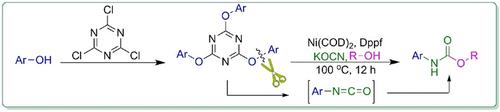
A simple and efficient domino reaction has been designed and employed for the one‐pot synthesis of N‐(hetero)aryl carbamates through the reaction between alcohols and in‐situ produced (hetero)aryl isocyanates in the presence of a nickel catalyst. The phenolic C−O bond was activated via the reaction of phenol with cyanuric chloride (2,4,6‐trichloro‐1,3,5‐triazine (TCT)) as an inexpensive and readily available reagent. This strategy provides practical access to N‐(hetero)aryl carbamates in good yields with high functional groups compatibility.
中文翻译:

镍催化氰基氯化物活化的氰酸盐和酚类化合物的镍催化合成N-(杂)芳基氨基甲酸酯
设计了一种简单有效的多米诺反应,并通过在镍催化剂存在下,醇与原位生成的(杂)芳基异氰酸酯之间的反应,单锅合成N-(杂)芳基氨基甲酸酯。通过苯酚与氰尿酰氯(2,4,6-三氯-1,3,5-三嗪(TCT))反应可以活化酚C-O键,这是一种廉价且易于获得的试剂。该策略提供了以高收率和高官能团相容性实际获得N-(杂)芳基氨基甲酸酯的方法。
更新日期:2020-07-19
中文翻译:

镍催化氰基氯化物活化的氰酸盐和酚类化合物的镍催化合成N-(杂)芳基氨基甲酸酯
设计了一种简单有效的多米诺反应,并通过在镍催化剂存在下,醇与原位生成的(杂)芳基异氰酸酯之间的反应,单锅合成N-(杂)芳基氨基甲酸酯。通过苯酚与氰尿酰氯(2,4,6-三氯-1,3,5-三嗪(TCT))反应可以活化酚C-O键,这是一种廉价且易于获得的试剂。该策略提供了以高收率和高官能团相容性实际获得N-(杂)芳基氨基甲酸酯的方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号