Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Free Thiol on Protein Adsorption to Gold Nanoparticles.
Langmuir ( IF 3.7 ) Pub Date : 2020-07-19 , DOI: 10.1021/acs.langmuir.0c01550
Olatunde Awotunde 1 , Samuel Okyem 1 , Rishika Chikoti 1 , Jeremy D Driskell 1
Langmuir ( IF 3.7 ) Pub Date : 2020-07-19 , DOI: 10.1021/acs.langmuir.0c01550
Olatunde Awotunde 1 , Samuel Okyem 1 , Rishika Chikoti 1 , Jeremy D Driskell 1
Affiliation
|
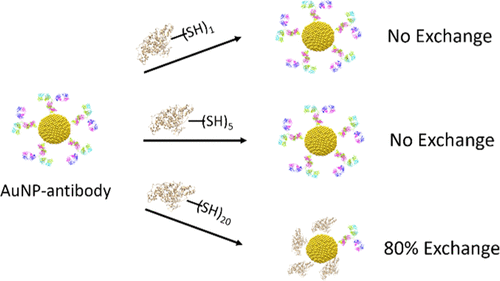
Protein–gold nanoparticle (AuNP) bioconjugates have many potential applications in nanomedicine. A thorough understanding of the interaction between the protein and the AuNP is critical to engineering these functional bioconjugates with desirable properties. In this work, we investigate the role of free thiols presented by the protein on the stability of the protein–AuNP conjugate. Human serum albumin (HSA) was modified with 2-iminothiolane (Traut’s reagent) to introduce additional thiols onto the protein surface, and three variants of HSA were synthesized to present 1, 5, and 20 free thiols by controlling the molar excess of the chemical modifier. Protein exchange studies on AuNPs were conducted using these HSA species and an IgG antibody which exhibited 10 free thiols. Antibody–AuNP conjugates were synthesized, purified, and dispersed in solutions containing each of the HSA species. No protein exchange was detected with the HSA or modified HSA containing 5 thiols; however, 85% of the antibody was displaced on the AuNP surface by the extensively thiolated HSA presenting 20 free thiols. Furthermore, the impact of the protein adsorption sequence was probed in which each of the HSA species were preadsorbed onto the AuNP and dispersed in a solution of antibody. The antibody fully displaced the HSA with a single thiol from the AuNP within 3 h, required 24 h to completely displace the modified HSA containing 5 thiols, and was unable to displace the modified HSA containing 20 thiols. These results indicate that the number of Au–S interactions governs the binding interaction between the protein and the AuNP. This work provides further insight into the protein–AuNP binding mechanism and identifies important design principles for engineered proteins to optimize bioconjugates.
中文翻译:

游离巯基在蛋白质吸附金纳米颗粒上的作用。
蛋白质-金纳米颗粒(AuNP)生物缀合物在纳米医学中具有许多潜在的应用。彻底了解蛋白质与AuNP之间的相互作用对于工程改造具有所需特性的这些功能性生物缀合物至关重要。在这项工作中,我们研究了蛋白质提供的游离硫醇对蛋白质-AuNP共轭物稳定性的作用。用2-亚氨基硫杂环戊烷(Traut's试剂)修饰人血清白蛋白(HSA),将另外的硫醇引入蛋白质表面,并通过控制化学物质的摩尔过量,合成了3种HSA变体,以提供1、5和20个游离硫醇修饰符。使用这些HSA物种和展示10种游离硫醇的IgG抗体对AuNPs进行蛋白质交换研究。抗体-AuNP共轭物经过合成,纯化,并分散在含有每种HSA物种的溶液中。没有检测到含有5个硫醇的HSA或修饰的HSA的蛋白质交换。然而,有85%的抗体被广泛巯基化的HSA取代,从而在AuNP表面置换了20个游离硫醇。此外,探测了蛋白质吸附顺序的影响,其中每种HSA物种都预先吸附在AuNP上并分散在抗体溶液中。抗体在3小时内用单硫醇从AuNP完全置换了HSA,需要24小时才能完全置换包含5个硫醇的修饰过的HSA,并且无法置换包含20个硫醇的修饰HSA。这些结果表明,Au–S相互作用的数量决定了蛋白质与AuNP之间的结合相互作用。
更新日期:2020-08-11
中文翻译:

游离巯基在蛋白质吸附金纳米颗粒上的作用。
蛋白质-金纳米颗粒(AuNP)生物缀合物在纳米医学中具有许多潜在的应用。彻底了解蛋白质与AuNP之间的相互作用对于工程改造具有所需特性的这些功能性生物缀合物至关重要。在这项工作中,我们研究了蛋白质提供的游离硫醇对蛋白质-AuNP共轭物稳定性的作用。用2-亚氨基硫杂环戊烷(Traut's试剂)修饰人血清白蛋白(HSA),将另外的硫醇引入蛋白质表面,并通过控制化学物质的摩尔过量,合成了3种HSA变体,以提供1、5和20个游离硫醇修饰符。使用这些HSA物种和展示10种游离硫醇的IgG抗体对AuNPs进行蛋白质交换研究。抗体-AuNP共轭物经过合成,纯化,并分散在含有每种HSA物种的溶液中。没有检测到含有5个硫醇的HSA或修饰的HSA的蛋白质交换。然而,有85%的抗体被广泛巯基化的HSA取代,从而在AuNP表面置换了20个游离硫醇。此外,探测了蛋白质吸附顺序的影响,其中每种HSA物种都预先吸附在AuNP上并分散在抗体溶液中。抗体在3小时内用单硫醇从AuNP完全置换了HSA,需要24小时才能完全置换包含5个硫醇的修饰过的HSA,并且无法置换包含20个硫醇的修饰HSA。这些结果表明,Au–S相互作用的数量决定了蛋白质与AuNP之间的结合相互作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号