当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adsorption Properties of SF6 on Zeolite NaY, 13X, Activated Carbon, and Silica Gel
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-07-17 , DOI: 10.1021/acs.jced.0c00348 Sirui Chen 1 , Yuanhui Shen 1 , Zhongbo Guan 1 , Bing Liu 1 , Zhongli Tang 1 , Donghui Zhang 1 , Bo Fu 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-07-17 , DOI: 10.1021/acs.jced.0c00348 Sirui Chen 1 , Yuanhui Shen 1 , Zhongbo Guan 1 , Bing Liu 1 , Zhongli Tang 1 , Donghui Zhang 1 , Bo Fu 2
Affiliation
|
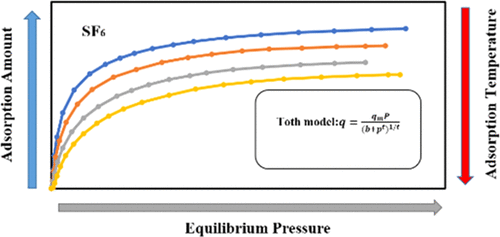
The SF6 adsorption isotherms on zeolite 13X, zeolite NaY, activated carbon, and silica gel were investigated over a temperature range of 298–358 K and at pressure from 0 to 1000 kPa. Experimental results demonstrated that the adsorption capacity of SF6 was in the order AC > NaY > 13X > silica gel under the same adsorption pressure and temperature conditions. Moreover, the equilibrium isotherm data of SF6 on different adsorbents were fitted with Langmuir, Langmuir–Freundlich, and Toth models. The agreement between the fitting isotherms and the experimental data increased with the increase of available fitting parameters in the equation of the isotherm model, and the Toth model was verified to be the best alternative for describing adsorption behaviors of SF6. Furthermore, the isosteric heats of adsorption of SF6 on adsorbents were calculated by the Clausius–Clapeyron equation. Heats of adsorption of SF6 on the four adsorbents first decreased and then increased with increasing SF6 loading.
中文翻译:

SF 6在NaY,13X沸石,活性炭和硅胶上的吸附性能
在298–358 K的温度范围和0至1000 kPa的压力下,研究了SF 6在13X沸石,NaY沸石,活性炭和硅胶上的吸附等温线。实验结果表明,在相同的吸附压力和温度条件下,SF 6的吸附能力为AC> NaY> 13X>硅胶。此外,用Langmuir,Langmuir-Freundlich和Toth模型拟合了不同吸附剂上SF 6的平衡等温线数据。拟合等温线与实验数据之间的一致性随着等温线模型方程中可用拟合参数的增加而增加,并且证明了Toth模型是描述SF吸附行为的最佳替代方法6。此外,通过克劳修斯-克拉珀龙方程计算了SF 6在吸附剂上的等规吸附热。SF 6在四种吸附剂上的吸附热首先随着SF 6负载的增加而降低,然后增加。
更新日期:2020-08-14
中文翻译:

SF 6在NaY,13X沸石,活性炭和硅胶上的吸附性能
在298–358 K的温度范围和0至1000 kPa的压力下,研究了SF 6在13X沸石,NaY沸石,活性炭和硅胶上的吸附等温线。实验结果表明,在相同的吸附压力和温度条件下,SF 6的吸附能力为AC> NaY> 13X>硅胶。此外,用Langmuir,Langmuir-Freundlich和Toth模型拟合了不同吸附剂上SF 6的平衡等温线数据。拟合等温线与实验数据之间的一致性随着等温线模型方程中可用拟合参数的增加而增加,并且证明了Toth模型是描述SF吸附行为的最佳替代方法6。此外,通过克劳修斯-克拉珀龙方程计算了SF 6在吸附剂上的等规吸附热。SF 6在四种吸附剂上的吸附热首先随着SF 6负载的增加而降低,然后增加。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号