Dyes and Pigments ( IF 4.1 ) Pub Date : 2020-07-18 , DOI: 10.1016/j.dyepig.2020.108672 Toshiyuki Uesaka , Tomoyuki Ishitani , Ryuhei Sawada , Takeshi Maeda , Shigeyuki Yagi
|
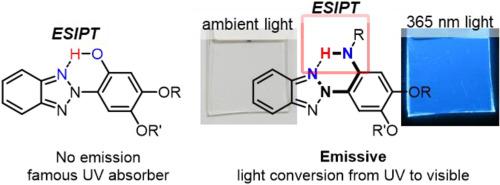
A series of 2-(2-phenyl)benzotriazole derivatives having amino, N-methyl amino, and acetamido groups on the ortho position of the phenyl group were synthesized to elucidate the effects of amino/amido substitutions on the molecular structures and photophysical properties. 1H NMR spectra and DFT calculations of the present dyes revealed the formation of N–H∙∙∙N intramolecular hydrogen bonds between benzotriazole and amino/amide groups in the ground state. Amino-functionalized dyes showed absorption bands that were assigned to HOMO-LUMO transition with a partial charge-transfer feature. The dye with an acetoamido group in which the acidity of NH proton was increased by the effect of electron-withdrawing acetyl groups exhibited absorption bands attributable to intramolecular hydrogen-bonded “closed” conformers in CHCl3 and non-hydrogen-bonded “open” conformers in DMSO. In comparison to 2-phenylbenzotriazole, which had no acidic proton involved in intramolecular hydrogen bonding, the present derivatives exhibited weak fluorescence emissions with quite low quantum yields, suggesting that non-radiative deactivation of ESIPT states also operated in the N–H∙∙∙N hydrogen bonded 2-phenylbenzotriazoles just as in O–H∙∙∙N intramolecular hydrogen-bonded 2-hydroxyphenylbenzotriazoles, which are known as UV absorbers. Taking a closer look at the fluorescence spectra, dyes with amino and N-methylamino groups showed faint but dual emissions from charge-transfer excited states in CHCl3 and blue-shifted single emission in DMSO. Fluorescence emissions of the acetamido-functionalized dye were unimodal in each solvent and stronger than those of amino-functionalized dyes. Thus, the fluorescence properties of these dyes depended on substitutions at amino groups. The luminescence of these derivatives was enhanced in the polymer matrix in which conformation changes and molecular motions were suppressed. Dye-doped polymer films have absorption bands in the UV range (360–400 nm) and emitted at the visible region (450–510 nm), and thus could be used as organic materials for light conversion films that convert UV light into visible light.
中文翻译:

具有分子内NH–··N氢键的荧光2-苯基-2 H-苯并三唑染料:质子给体取代基的合成和荧光性质的调控
合成了一系列在苯基邻位具有氨基,N-甲基氨基和乙酰氨基的2-(2-苯基)苯并三唑衍生物,以阐明氨基/酰胺取代对分子结构和光物理性质的影响。1个本染料的1 H NMR光谱和DFT计算表明,基态下苯并三唑与氨基/酰胺基之间形成了N–H∙∙∙N分子内氢键。氨基官能化的染料显示出吸收带,该吸收带被分配为具有部分电荷转移功能的HOMO-LUMO跃迁。带有乙酰酰胺基的染料,其中NH质子的酸性由于吸电子乙酰基的作用而增加,在CHCl 3中表现出归因于分子内氢键“封闭”构象异构体的吸收带以及DMSO中非氢键键合的“开放”构象体。与没有酸性质子参与分子内氢键结合的2-苯基苯并三唑相比,目前的衍生物显示出弱的荧光发射,具有相当低的量子产率,这表明ESIPT态的非辐射失活也在N–H∙∙∙中起作用N氢键合的2-苯基苯并三唑与O–H∙∙∙N N分子内氢键合的2-羟基苯基苯并三唑一样,被称为紫外线吸收剂。仔细观察荧光光谱,带有氨基和N-甲基氨基的染料在CHCl 3中显示出微弱但由电荷转移激发态产生的双重发射DMSO中的蓝移单发射。乙酰氨基官能化染料的荧光发射在每种溶剂中都是单峰的,并且比氨基官能化染料的荧光发射更强。因此,这些染料的荧光性质取决于氨基上的取代。这些衍生物的发光在聚合物基质中得到增强,其中构象变化和分子运动得到抑制。染料掺杂的聚合物薄膜具有在紫外线范围(360–400 nm)内的吸收带,并在可见光区域(450–510 nm)发出,因此可以用作将紫外线转换为可见光的光转换薄膜的有机材料。 。






























 京公网安备 11010802027423号
京公网安备 11010802027423号