当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrate Stability Conditions of CO2 + TBPB + Cyclopentane + Water System: Experimental Measurements and Thermodynamic Modeling
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-07-17 , DOI: 10.1021/acs.jced.0c00389 Samira Mohammadi 1 , Hassan Pahlavanzadeh 1 , Amir H. Mohammadi 2 , Hussein Hassan 1 , Sepideh Nouri 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-07-17 , DOI: 10.1021/acs.jced.0c00389 Samira Mohammadi 1 , Hassan Pahlavanzadeh 1 , Amir H. Mohammadi 2 , Hussein Hassan 1 , Sepideh Nouri 1
Affiliation
|
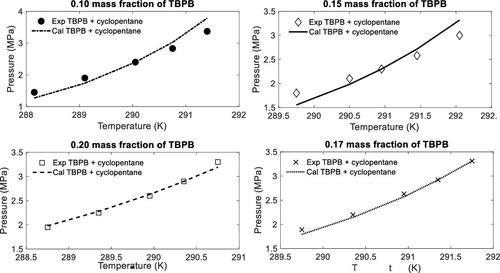
The current study provides experimental data and thermodynamic modeling of hydrate stability conditions of CO2 + tetra n-butylphosphonium bromide (TBPB) + cyclopentane (CP) + water system. The experimental results are presented from (288 to 292) K and (1.45 to 3.30) MPa at 0.10, 0.15, 0.17, and 0.20 mass fractions of TBPB in aqueous solutions. To measure the experimental data, an isochoric step-heating pressure-search method was applied. The validity of the method used in the current study was evaluated by regenerating previously reported experimental data on hydrate dissociation conditions of the carbon dioxide + TBPB + water system. The experimental results reveal that mixed promoters of TBPB + CP have the promotion effect compared to carbon dioxide + water and carbon dioxide + TBPB + water systems and the inhibition impact compared to the carbon dioxide + CP + water system; however, the inhibition effect is not considerable. Furthermore, a thermodynamic model was developed to predict/represent hydrate stability conditions. Nonrandom two-liquid (NRTL) activity coefficient model, e-NRTL activity coefficient model, and Peng–Robinson equation of state together with van der Waals–Platteeuw theory are applied to model the organic-rich phase and nonideality in the aqueous/liquid phase, vapor/gas phase, and hydrate phase, respectively. Experimental hydrate stability conditions at 0.10, 0.15, and 0.20 TBPB mass fractions were used to obtain the parameters of the thermodynamic model. Using the obtained parameters, the hydrate dissociation conditions at 0.17 mass fraction of TBPB were predicted. It is indicated that the thermodynamic model could predict the experimental data satisfactorily. The average relative deviation (ARD %) of the model results for hydrate dissociation pressures is about 5.4%.
中文翻译:

CO 2 + TBPB +环戊烷+水系统的水合物稳定性条件:实验测量和热力学模型
目前的研究提供了实验数据和CO的水合物稳定条件热力学模型2 +四Ñ-丁基溴化phosph(TBPB)+环戊烷(CP)+水系统。在TBPB的质量分数为0.10、0.15、0.17和0.20的水溶液中,实验结果显示为(288至292)K和(1.45至3.30)MPa。为了测量实验数据,应用了等分步加热压力搜索方法。通过再生先前报道的有关二氧化碳+ TBPB +水系统的水合物解离条件的实验数据,评估了本研究中使用的方法的有效性。实验结果表明,与二氧化碳+水和二氧化碳+ TBPB +水体系相比,TBPB + CP的混合促进剂具有促进作用,与二氧化碳+ CP +水体系相比具有抑制作用。但是,抑制效果不明显。此外,开发了一个热力学模型来预测/表示水合物的稳定性条件。非随机两液(NRTL)活度系数模型,e-NRTL活度系数模型,Peng-Robinson状态方程和Van der Waals-Platteeuw理论一起用于模拟富有机相和水/液相中的非理想状态,蒸气/气相和水合物相。使用在0.10、0.15和0.20 TBPB质量分数下的实验水合物稳定性条件来获得热力学模型的参数。使用获得的参数,预测TBPB为0.17质量分数时的水合物解离条件。结果表明,热力学模型可以较好地预测实验数据。
更新日期:2020-08-14
中文翻译:

CO 2 + TBPB +环戊烷+水系统的水合物稳定性条件:实验测量和热力学模型
目前的研究提供了实验数据和CO的水合物稳定条件热力学模型2 +四Ñ-丁基溴化phosph(TBPB)+环戊烷(CP)+水系统。在TBPB的质量分数为0.10、0.15、0.17和0.20的水溶液中,实验结果显示为(288至292)K和(1.45至3.30)MPa。为了测量实验数据,应用了等分步加热压力搜索方法。通过再生先前报道的有关二氧化碳+ TBPB +水系统的水合物解离条件的实验数据,评估了本研究中使用的方法的有效性。实验结果表明,与二氧化碳+水和二氧化碳+ TBPB +水体系相比,TBPB + CP的混合促进剂具有促进作用,与二氧化碳+ CP +水体系相比具有抑制作用。但是,抑制效果不明显。此外,开发了一个热力学模型来预测/表示水合物的稳定性条件。非随机两液(NRTL)活度系数模型,e-NRTL活度系数模型,Peng-Robinson状态方程和Van der Waals-Platteeuw理论一起用于模拟富有机相和水/液相中的非理想状态,蒸气/气相和水合物相。使用在0.10、0.15和0.20 TBPB质量分数下的实验水合物稳定性条件来获得热力学模型的参数。使用获得的参数,预测TBPB为0.17质量分数时的水合物解离条件。结果表明,热力学模型可以较好地预测实验数据。































 京公网安备 11010802027423号
京公网安备 11010802027423号