当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly Diastereoselective Synthesis of 2,2-Disubstituted Cyclopentane-1,3-diols via Stepwise Ketone Reduction Enabling Concise Chirality Construction.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-07-15 , DOI: 10.1021/acs.joc.0c00851 Jingyao Gong 1, 2 , Jianjiong Li 2 , Xi Chen 2 , Hongliu Zhang 2 , Liangyan Zhu 2 , Dandan Bu 2 , Qin Wang 1 , Jinhui Feng 2 , Qiaqing Wu 2 , Dunming Zhu 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-07-15 , DOI: 10.1021/acs.joc.0c00851 Jingyao Gong 1, 2 , Jianjiong Li 2 , Xi Chen 2 , Hongliu Zhang 2 , Liangyan Zhu 2 , Dandan Bu 2 , Qin Wang 1 , Jinhui Feng 2 , Qiaqing Wu 2 , Dunming Zhu 2
Affiliation
|
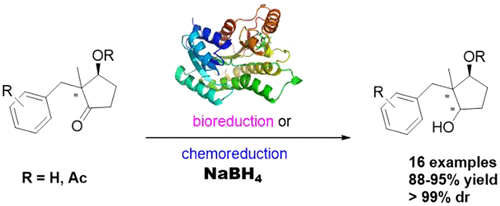
A series of 2,2-disubstituted trans,cis-cyclopentane-1,3-diols were synthesized in >99% dr through enzymatic reduction of enantiopure 2,2-disubstituted 3-hydroxycyclopentane-1-ones, which were prepared by highly stereoselective enzymatic reduction of the corresponding cyclodiketones. For 2-benzyl-2-methyl-3-oxocyclopentyl acetate, acetylation of the hydroxyl group significantly affected the reduction stereoselectivity, giving trans,cis-, trans,trans-, and cis,cis-2-benzyl-2-methyl-cyclopentane-1,3-diols in stereomerically pure form. This efficient and environmentally friendly method provides a practical approach to the synthesis of these chiral building blocks in single stereoisomeric form, demonstrating the power of biocatalysis in the concise chirality construction of complex chiral molecules.
中文翻译:

通过逐步酮还原实现高度手性的高度非对映选择性合成2,2-二取代的环戊烷-1,3-二醇。
通过对映体纯的2,2-二取代的3-羟基环戊烷-1-酮的酶促还原反应,以> 99%dr合成了一系列2,2-二取代的反式,顺式-s-环戊烷-1,3-二醇。相应环二酮的立体选择性酶促还原。2-苄基-2-甲基-3-氧代环戊基乙酸酯,羟基的乙酰化显著影响立体选择性还原,得到反式,CI仲- ,反式,反式- ,和顺式,顺式立体异构纯形式的-2-苄基-2-甲基-环戊烷-1,3-二醇。这种高效且环保的方法为单一立体异构形式的这些手性结构单元的合成提供了一种实用的方法,证明了在复杂手性分子的简明手性结构中生物催化的力量。
更新日期:2020-08-08
中文翻译:

通过逐步酮还原实现高度手性的高度非对映选择性合成2,2-二取代的环戊烷-1,3-二醇。
通过对映体纯的2,2-二取代的3-羟基环戊烷-1-酮的酶促还原反应,以> 99%dr合成了一系列2,2-二取代的反式,顺式-s-环戊烷-1,3-二醇。相应环二酮的立体选择性酶促还原。2-苄基-2-甲基-3-氧代环戊基乙酸酯,羟基的乙酰化显著影响立体选择性还原,得到反式,CI仲- ,反式,反式- ,和顺式,顺式立体异构纯形式的-2-苄基-2-甲基-环戊烷-1,3-二醇。这种高效且环保的方法为单一立体异构形式的这些手性结构单元的合成提供了一种实用的方法,证明了在复杂手性分子的简明手性结构中生物催化的力量。








































 京公网安备 11010802027423号
京公网安备 11010802027423号