当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ru-Catalyzed Geminal Hydroboration of Silyl Alkynes via a New gem-Addition Mechanism
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-15 , DOI: 10.1021/jacs.0c05334 Qiang Feng 1 , Haonan Wu 2, 3 , Xin Li 2 , Lijuan Song 4 , Lung Wa Chung 2 , Yun-Dong Wu 3, 4, 5 , Jianwei Sun 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-07-15 , DOI: 10.1021/jacs.0c05334 Qiang Feng 1 , Haonan Wu 2, 3 , Xin Li 2 , Lijuan Song 4 , Lung Wa Chung 2 , Yun-Dong Wu 3, 4, 5 , Jianwei Sun 1
Affiliation
|
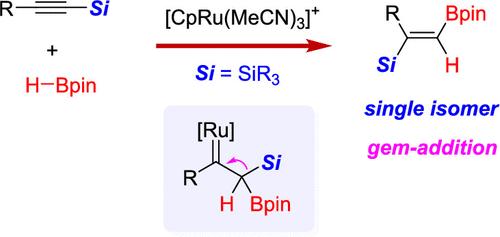
While 1,2-addition represents the most common mode of alkyne hydroboration, herein we describe a new 1,1-hydroboration mode. It is the first demonstration of gem-(H,B) addition to an alkyne triple bond. With the superior [CpRu(MeCN)3]PF6 catalyst, a range of silyl alkynes reacted efficiently with HBpin under mild conditions to form various synthetically useful silyl vinyl boronates with complete stereoselectivity and broad functional group compatibility. An extension to germanyl alkynes as well as the hydrosilylation of alkynyl boronates toward the same type products was also achieved. Mechanistically, this process features a new pathway featuring gem-(H,B) addition to form the key α-boryl-α-silyl Ru-carbene intermediate followed by silyl migration. It is believed that the orbital interaction between boron and Cβ in the coplanar relationship between the boron atom and the ruthenacyclopropene ring preceding boron migration is responsible for the new reactivity. Control experiments and DFT (including molecular dynamics) calculations provided important insights into the mechanism, which excluded the involvement of metal vinylidene intermediate. This study represents a new step forward not only for alkyne hydroboration, but also for other geminal alkyne additions.
中文翻译:

通过新的宝石加成机制 Ru 催化甲硅烷基炔烃的 Geminal 硼氢化
虽然 1,2-加成代表最常见的炔烃硼氢化模式,但在此我们描述了一种新的 1,1-硼氢化模式。这是第一次展示了 gem-(H,B) 加成到炔三键上。凭借优异的 [CpRu(MeCN)3]PF6 催化剂,一系列甲硅烷基炔烃在温和条件下与 HBpin 有效反应,形成各种合成有用的甲硅烷基乙烯基硼酸酯,具有完全立体选择性和广泛的官能团兼容性。还实现了对锗基炔烃的扩展以及炔基硼酸酯向相同类型产品的氢化硅烷化。从机制上讲,该过程具有一种新途径,其特征是 gem-(H,B) 添加形成关键的 α-硼基-α-甲硅烷基 Ru-卡宾中间体,然后是甲硅烷基迁移。据信,硼和 Cβ 之间的轨道相互作用在硼原子和硼迁移之前的钌环丙烯环之间的共面关系中是新反应性的原因。对照实验和 DFT(包括分子动力学)计算提供了对该机制的重要见解,排除了金属亚乙烯基中间体的参与。这项研究不仅代表炔烃硼氢化作用向前迈出的新一步,也代表了其他成对炔烃的添加。
更新日期:2020-07-15
中文翻译:

通过新的宝石加成机制 Ru 催化甲硅烷基炔烃的 Geminal 硼氢化
虽然 1,2-加成代表最常见的炔烃硼氢化模式,但在此我们描述了一种新的 1,1-硼氢化模式。这是第一次展示了 gem-(H,B) 加成到炔三键上。凭借优异的 [CpRu(MeCN)3]PF6 催化剂,一系列甲硅烷基炔烃在温和条件下与 HBpin 有效反应,形成各种合成有用的甲硅烷基乙烯基硼酸酯,具有完全立体选择性和广泛的官能团兼容性。还实现了对锗基炔烃的扩展以及炔基硼酸酯向相同类型产品的氢化硅烷化。从机制上讲,该过程具有一种新途径,其特征是 gem-(H,B) 添加形成关键的 α-硼基-α-甲硅烷基 Ru-卡宾中间体,然后是甲硅烷基迁移。据信,硼和 Cβ 之间的轨道相互作用在硼原子和硼迁移之前的钌环丙烯环之间的共面关系中是新反应性的原因。对照实验和 DFT(包括分子动力学)计算提供了对该机制的重要见解,排除了金属亚乙烯基中间体的参与。这项研究不仅代表炔烃硼氢化作用向前迈出的新一步,也代表了其他成对炔烃的添加。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号