当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Potent and Orally Available Bicyclo[1.1.1]pentane-Derived Indoleamine-2,3-dioxygenase 1 (IDO1) Inhibitors.
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-07-15 , DOI: 10.1021/acsmedchemlett.0c00195 Qinglin Pu 1 , Hongjun Zhang 1 , Liangqin Guo 1 , Mangeng Cheng 1 , Amy C Doty 1 , Heidi Ferguson 1 , Xavier Fradera 1 , Charles A Lesburg 1 , Meredeth A McGowan 1 , J Richard Miller 1 , Prasanthi Geda 1 , Xuelei Song 1 , Karin Otte 1 , Nunzio Sciammetta 1 , Nicolas Solban 1 , Wensheng Yu 2 , David L Sloman 1 , Hua Zhou 1 , Alfred Lammens 3 , Lars Neumann 3 , David Jonathan Bennett 1 , Alexander Pasternak 1 , Yongxin Han 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-07-15 , DOI: 10.1021/acsmedchemlett.0c00195 Qinglin Pu 1 , Hongjun Zhang 1 , Liangqin Guo 1 , Mangeng Cheng 1 , Amy C Doty 1 , Heidi Ferguson 1 , Xavier Fradera 1 , Charles A Lesburg 1 , Meredeth A McGowan 1 , J Richard Miller 1 , Prasanthi Geda 1 , Xuelei Song 1 , Karin Otte 1 , Nunzio Sciammetta 1 , Nicolas Solban 1 , Wensheng Yu 2 , David L Sloman 1 , Hua Zhou 1 , Alfred Lammens 3 , Lars Neumann 3 , David Jonathan Bennett 1 , Alexander Pasternak 1 , Yongxin Han 1
Affiliation
|
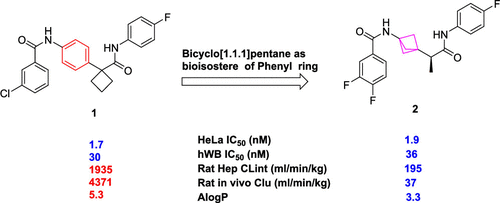
Indoleamine-2,3-dioxygenase 1 (IDO1) inhibition and its combination with immune checkpoint inhibitors like pembrolizumab have drawn considerable attention from both academia and the pharmaceutical industry. Here, we describe the discovery of a novel class of highly potent IDO1 heme-displacing inhibitors featuring a unique bicyclo[1.1.1]pentane motif. Compound 1, evolving from an ALIS (automated ligand identification system) hit, exhibited excellent potency but lacked the desired pharmacokinetic profile due to extensive amide hydrolysis of the benzamide moiety. Replacing the central phenyl ring in 1 with a bicyclo[1.1.1]pentane bioisostere effectively circumvented the amide hydrolysis issue, resulting in the discovery of compound 2 with a favorable overall profile such as excellent potency, selectivity, pharmacokinetics, and a low predicted human dose.
中文翻译:

发现有效且可口服的双环 [1.1.1] 戊烷衍生的吲哚胺-2,3-双加氧酶 1 (IDO1) 抑制剂。
Indoleamine-2,3-dioxygenase 1 (IDO1) 抑制及其与免疫检查点抑制剂(如派姆单抗)的结合引起了学术界和制药业的广泛关注。在这里,我们描述了一类新型高效 IDO1 血红素置换抑制剂的发现,该抑制剂具有独特的双环 [1.1.1] 戊烷基序。从ALIS(自动配体识别系统)命中演化而来的化合物1表现出优异的效力,但由于苯甲酰胺部分的广泛酰胺水解而缺乏所需的药代动力学特征。更换在中央苯环1用双环[1.1.1]戊烷生物电子等排有效规避酰胺水解问题,从而导致化合物的发现2 具有良好的总体特征,例如出色的效力、选择性、药代动力学和较低的预测人体剂量。
更新日期:2020-08-14
中文翻译:

发现有效且可口服的双环 [1.1.1] 戊烷衍生的吲哚胺-2,3-双加氧酶 1 (IDO1) 抑制剂。
Indoleamine-2,3-dioxygenase 1 (IDO1) 抑制及其与免疫检查点抑制剂(如派姆单抗)的结合引起了学术界和制药业的广泛关注。在这里,我们描述了一类新型高效 IDO1 血红素置换抑制剂的发现,该抑制剂具有独特的双环 [1.1.1] 戊烷基序。从ALIS(自动配体识别系统)命中演化而来的化合物1表现出优异的效力,但由于苯甲酰胺部分的广泛酰胺水解而缺乏所需的药代动力学特征。更换在中央苯环1用双环[1.1.1]戊烷生物电子等排有效规避酰胺水解问题,从而导致化合物的发现2 具有良好的总体特征,例如出色的效力、选择性、药代动力学和较低的预测人体剂量。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号