当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One‐pot, Direct Synthesis of 3‐Hydroxy‐3‐aryl‐1‐indanones and their 2‐Benzylidene Derivatives from 2‐Alkynylbenzophenones
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-07-15 , DOI: 10.1002/slct.202001104 Jampani Santhi 1 , Beeraiah Baire 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-07-15 , DOI: 10.1002/slct.202001104 Jampani Santhi 1 , Beeraiah Baire 1
Affiliation
|
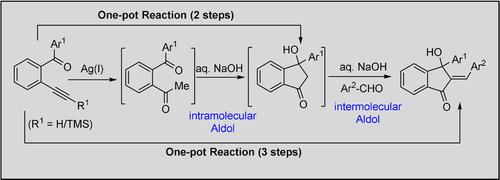
A rapid and efficient one‐pot strategy for the synthesis of 3‐hydroxy‐3‐aryl‐1‐indanones, directly from the corresponding 2‐alkynylbezophenones via a sequential carbonyl directed regioselective hydration followed by an intramolecular Aldol reaction has been reported. Further this strategy has also been extended for the efficient conversion of these 3‐hydroxy‐3‐aryl‐1‐indanone units into biologically important 2‐benzylidene‐3‐hydroxy‐3‐aryl‐1‐indanone derivatives, employing an intermolecular Aldol condensation reaction, both in a stepwise manner as well as one pot strategy. The process exhibits very good substrate scope and high reaction yields under mild reaction conditions.
中文翻译:

从2-炔基二苯甲酮一锅直接合成3-羟基-3-芳基-1-茚满酮及其2-苄叉基衍生物
已经报道了一种快速有效的一锅策略,可以直接从相应的2-炔基二苯甲酮通过连续的羰基定向的区域选择性水合反应,然后进行分子内的Aldol反应合成3-羟基-3-芳基-1-茚满酮。此外,通过分子间的Aldol缩合,该策略也得到了扩展,可以有效地将这些3-羟基-3-芳基-1-茚满酮单元转化为生物学上重要的2-苄叉基-3-羟基-3-芳基-1-茚满酮衍生物。反应,既可以逐步进行,也可以采取一锅策略。该方法在温和的反应条件下具有很好的底物范围和高的反应产率。
更新日期:2020-07-15
中文翻译:

从2-炔基二苯甲酮一锅直接合成3-羟基-3-芳基-1-茚满酮及其2-苄叉基衍生物
已经报道了一种快速有效的一锅策略,可以直接从相应的2-炔基二苯甲酮通过连续的羰基定向的区域选择性水合反应,然后进行分子内的Aldol反应合成3-羟基-3-芳基-1-茚满酮。此外,通过分子间的Aldol缩合,该策略也得到了扩展,可以有效地将这些3-羟基-3-芳基-1-茚满酮单元转化为生物学上重要的2-苄叉基-3-羟基-3-芳基-1-茚满酮衍生物。反应,既可以逐步进行,也可以采取一锅策略。该方法在温和的反应条件下具有很好的底物范围和高的反应产率。






























 京公网安备 11010802027423号
京公网安备 11010802027423号