Molecular Therapy - Methods & Clinical Development ( IF 4.6 ) Pub Date : 2020-07-15 , DOI: 10.1016/j.omtm.2020.07.010
Stefan Radtke 1, 2 , Dnyanada Pande 1, 2 , Margaret Cui 1, 2 , Anai M Perez 1, 2 , Yan-Yi Chan 1, 2 , Mark Enstrom 1, 2 , Stefanie Schmuck 1, 2 , Andrew Berger 2 , Tom Eunson 2 , Jennifer E Adair 1, 2, 3 , Hans-Peter Kiem 1, 2, 4, 5
|
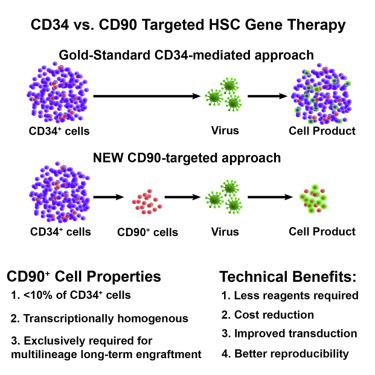
Hematopoietic stem cell (HSC) gene therapy has the potential to cure many genetic, malignant, and infectious diseases. We have shown in a nonhuman primate gene therapy and transplantation model that the CD34+CD90+ cell fraction was exclusively responsible for multilineage engraftment and hematopoietic reconstitution. In this study, we show the translational potential of this HSC-enriched CD34 subset for lentivirus-mediated gene therapy. Alternative HSC enrichment strategies include the purification of CD133+ cells or CD38low/– subsets of CD34+ cells from human blood products. We directly compared these strategies to the isolation of CD90+ cells using a good manufacturing practice (GMP) grade flow-sorting protocol with clinical applicability. We show that CD90+ cell selection results in about 30-fold fewer target cells in comparison to CD133+ or CD38low/– CD34+ hematopoietic stem and progenitor cell (HSPC) subsets without compromising the engraftment potential in vivo. Single-cell RNA sequencing confirmed nearly complete depletion of lineage-committed progenitor cells in CD90+ fractions compared to alternative selections. Importantly, lentiviral transduction efficiency in purified CD90+ cells resulted in up to 3-fold higher levels of engrafted gene-modified blood cells. These studies should have important implications for the manufacturing of patient-specific HSC gene therapy and gene-engineered cell products.
中文翻译:

人 CD34+CD90+ HSC 的纯化减少了靶细胞数量并改善了基因治疗的慢病毒转导。
造血干细胞 (HSC) 基因疗法具有治愈许多遗传、恶性和传染性疾病的潜力。我们已经在非人类灵长类动物基因治疗和移植模型中表明,CD34 + CD90 +细胞部分专门负责多系移植和造血重建。在这项研究中,我们展示了这种富含 HSC 的 CD34 子集在慢病毒介导的基因治疗中的转化潜力。替代的 HSC 富集策略包括从人类血液制品中纯化 CD133 +细胞或 CD38低/- CD34 +细胞亚群。我们直接将这些策略与 CD90 +的分离进行了比较使用具有临床适用性的良好生产规范 (GMP) 级流式分选方案的细胞。我们表明,与 CD133 +或 CD38低/– CD34 +造血干细胞和祖细胞 (HSPC) 亚群相比,CD90 +细胞选择导致靶细胞减少约 30 倍,而不会影响体内植入潜力。单细胞 RNA 测序证实,与替代选择相比,CD90 +级分中的谱系定向祖细胞几乎完全耗尽。重要的是,纯化 CD90 + 中的慢病毒转导效率细胞导致移植的基因修饰血细胞水平高出 3 倍。这些研究应该对制造患者特异性 HSC 基因疗法和基因工程细胞产品具有重要意义。

































 京公网安备 11010802027423号
京公网安备 11010802027423号