当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biocatalytic Access to 1,4-Diazepanes via Imine Reductase-Catalyzed Intramolecular Asymmetric Reductive Amination
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-07-13 , DOI: 10.1021/acscatal.0c02400 Zefei Xu 1, 2 , Peiyuan Yao 1, 2 , Xiang Sheng 3 , Jinlong Li 2 , Jianjiong Li 2 , Shanshan Yu 2 , Jinhui Feng 2 , Qiaqing Wu 1, 2 , Dunming Zhu 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-07-13 , DOI: 10.1021/acscatal.0c02400 Zefei Xu 1, 2 , Peiyuan Yao 1, 2 , Xiang Sheng 3 , Jinlong Li 2 , Jianjiong Li 2 , Shanshan Yu 2 , Jinhui Feng 2 , Qiaqing Wu 1, 2 , Dunming Zhu 1, 2
Affiliation
|
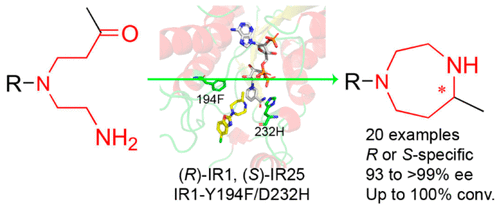
An enzymatic intramolecular asymmetric reductive amination has been developed for the synthesis of chiral 1,4-diazepanes. Several enantiocomplementary IREDs were identified for the synthesis of (R)- and (S)-5-chloro-2-(5-methyl-1,4-diazepan-1-yl)benzo[d]oxazole with high enantioselectivity. The catalytic efficiency of (R)-selective IRED from Leishmania major (IR1) and (S)-selective IRED from Micromonospora echinaurantiaca (IR25) was 0.027 and 0.962 s–1 mM–1, respectively. To further improve the catalytic efficiency of IR1, its double mutant Y194F/D232H was identified by saturation mutagenesis and iterative combinatorial mutagenesis, which exhibited 61-fold in the catalytic efficiency relative to that of wild-type enzyme. The density functional calculations and molecular dynamics simulations provided some insights into the molecular basis for the improved activity of mutant Y194F/D232H. Furthermore, Y194F/D232H and IR25 were applied to access a range of different substituted 1,4-diazepanes with high enantiomeric excess (from 93 to >99%). This study offers an effective method for construction of chiral 1,4-diazepanes of pharmaceutical importance via imine reductase-catalyzed intramolecular reductive amination of the corresponding aminoketones.
中文翻译:

通过亚胺还原酶催化的分子内不对称还原胺化对1,4-二氮杂酮的生物催化访问
已经开发出一种酶促分子内不对称还原胺化用于手性1,4-二氮杂dia的合成。鉴定了几种对映体互补的IRED,以高对映选择性合成(R)-和(S)-5-氯-2-(5-甲基-1,4-二氮杂-1-基)苯并[ d ]恶唑。大利什曼原虫(IR1)的(R)选择性IRED和紫锥菊单胞菌(IR25)的(S)选择性IRED的催化效率分别为0.027和0.962 s –1 mM –1, 分别。为了进一步提高IR1的催化效率,通过饱和诱变和迭代组合诱变鉴定了它的双重突变体Y194F / D232H,其催化效率是野生型酶的61倍。密度泛函计算和分子动力学模拟为突变体Y194F / D232H的活性提高提供了分子基础的一些见识。此外,使用Y194F / D232H和IR25来获得对映体过量高(从93%到> 99%)的一系列不同的取代1,4-二氮杂苯。这项研究提供了一种有效的方法,通过亚胺还原酶催化的相应氨基酮的分子内还原胺化来构建具有重要药学意义的手性1,4-二氮杂ze。
更新日期:2020-08-08
中文翻译:

通过亚胺还原酶催化的分子内不对称还原胺化对1,4-二氮杂酮的生物催化访问
已经开发出一种酶促分子内不对称还原胺化用于手性1,4-二氮杂dia的合成。鉴定了几种对映体互补的IRED,以高对映选择性合成(R)-和(S)-5-氯-2-(5-甲基-1,4-二氮杂-1-基)苯并[ d ]恶唑。大利什曼原虫(IR1)的(R)选择性IRED和紫锥菊单胞菌(IR25)的(S)选择性IRED的催化效率分别为0.027和0.962 s –1 mM –1, 分别。为了进一步提高IR1的催化效率,通过饱和诱变和迭代组合诱变鉴定了它的双重突变体Y194F / D232H,其催化效率是野生型酶的61倍。密度泛函计算和分子动力学模拟为突变体Y194F / D232H的活性提高提供了分子基础的一些见识。此外,使用Y194F / D232H和IR25来获得对映体过量高(从93%到> 99%)的一系列不同的取代1,4-二氮杂苯。这项研究提供了一种有效的方法,通过亚胺还原酶催化的相应氨基酮的分子内还原胺化来构建具有重要药学意义的手性1,4-二氮杂ze。































 京公网安备 11010802027423号
京公网安备 11010802027423号