当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of cis-Decalin Derivatives by the Inverse-Electron-Demand Diels-Alder Reaction of 2-Pyrones.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-13 , DOI: 10.1002/anie.202006841 Xu-Ge Si 1 , Zhi-Mao Zhang 1 , Cheng-Gong Zheng 1 , Zhan-Ting Li 1 , Quan Cai 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-13 , DOI: 10.1002/anie.202006841 Xu-Ge Si 1 , Zhi-Mao Zhang 1 , Cheng-Gong Zheng 1 , Zhan-Ting Li 1 , Quan Cai 1
Affiliation
|
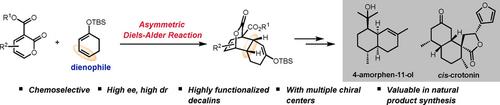
A novel strategy for the synthesis of cis‐decalins by an ytterbium‐catalyzed asymmetric inverse‐electron‐demand Diels–Alder reaction of 2‐pyrones and silyl cyclohexadienol ethers is reported here. A broad range of synthetically important cis‐decalin derivatives with multiple contiguous stereogenic centers and functionalities are obtained in good yields and stereoselectivities. A full set of diastereomeric substituted cis‐decalin motifs are readily accessible by tuning the absolute configurations of substituted silyl cyclohexadienol ethers (R or S) as well as the ligands (R or S). The synthetic potential is showcased by the enantioselective total synthesis of 4‐amorphen‐11‐ol, and further demonstrated by the first total synthesis of cis‐crotonin.
中文翻译:

通过2-吡喃酮的电子反需求Diels-Alder反应对映体合成顺式十氢化萘衍生物。
本文报道了由by催化的2-吡喃酮和甲硅烷基环己二烯醚的不对称逆电子需求的Diels-Alder反应合成顺式十萘烷的新策略。具有良好的收率和立体选择性,可获得具有多个连续的立体中心和功能性的广泛的重要合成顺式癸萘衍生物。通过调整取代的甲硅烷基环己二烯醚(R或S)以及配体(R或S)的绝对构型,可以轻松获得全套非对映异构取代的顺式-十氢萘基序)。合成潜力通过4-amorphen-11-ol的对映选择性全合成得到展示,并通过首次顺式-巴豆丁的全合成得到进一步证明。
更新日期:2020-07-13
中文翻译:

通过2-吡喃酮的电子反需求Diels-Alder反应对映体合成顺式十氢化萘衍生物。
本文报道了由by催化的2-吡喃酮和甲硅烷基环己二烯醚的不对称逆电子需求的Diels-Alder反应合成顺式十萘烷的新策略。具有良好的收率和立体选择性,可获得具有多个连续的立体中心和功能性的广泛的重要合成顺式癸萘衍生物。通过调整取代的甲硅烷基环己二烯醚(R或S)以及配体(R或S)的绝对构型,可以轻松获得全套非对映异构取代的顺式-十氢萘基序)。合成潜力通过4-amorphen-11-ol的对映选择性全合成得到展示,并通过首次顺式-巴豆丁的全合成得到进一步证明。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号