Electrochimica Acta ( IF 5.5 ) Pub Date : 2020-07-14 , DOI: 10.1016/j.electacta.2020.136706
Jing Wang , Tong Zheng , Huiling Liu , Gang Wang , Yanxiang Zhang , Chen Cai
|
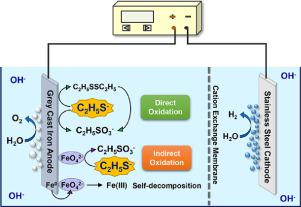
In this study, the electrochemical generation of ferrate (Fe(VI)) from grey cast iron anode in sodium hydroxide (NaOH) solution was utilized in situ to degrade the malodorous pollutant of ethanethiol (C2H5SH). During electrolysis, C2H5SH was oxidized by the combination of direct anodic oxidation and Fe(VI) oxidation (indirect oxidation). The direct anodic oxidation process, which played an important role in the decontamination process but was often disregarded in related studies, was investigated in detail. The electrochemical generation of Fe(VI) was investigated, and the maximum current efficiency of Fe(VI) generation was obtained in 14 M NaOH solution. The voltammetry studies of the grey cast iron anode in NaOH solutions containing and without C2H5SH revealed that the dissolution of anode was improved in the presence of C2H5SH. A decrease in NaOH concentration increased the contribution of direct anodic oxidation but decreased the electrochemical generation of Fe(VI). C2H5SH was degraded on the grey cast iron anode at a higher rate in less concentrated NaOH solution (k = 4.15 × 10−2 min−1 in 2 M NaOH solution), which could be ascribed to the enhancement in direct anodic oxidation as well as in the oxidizing power of Fe(VI). Higher current density promoted the degradation rate of C2H5SH in both direct and indirect oxidation processes, but it also decreased the current efficiency due to the improvement of the side reactions. Additionally, the in-situ and on-line applications of the electrochemical generated Fe(VI) in recent studies were presented and discussed. This study provides a comprehensive understanding of C2H5SH oxidation on grey cast iron anode, and offers a feasible electrochemical scrubbing approach using grey cast iron as the anode for C2H5SH odors control.
中文翻译:

灰溶液中灰铸铁阳极上乙硫醇的直接和间接电化学氧化
在这项研究中,原位利用灰铸铁阳极在氢氧化钠(NaOH)溶液中电化学生成高铁酸盐(Fe(VI))来降解乙硫醇(C 2 H 5 SH)的恶臭污染物。电解过程中,C 2 H 5SH通过直接阳极氧化和Fe(VI)氧化(间接氧化)的组合被氧化。详细研究了直接阳极氧化过程,该过程在去污过程中起着重要作用,但在相关研究中经常被忽略。研究了Fe(VI)的电化学生成,并在14 M NaOH溶液中获得了Fe(VI)生成的最大电流效率。灰铸铁阳极在含和不含C 2 H 5 SH的NaOH溶液中的伏安研究表明,在C 2 H 5存在下,阳极的溶解度得到了改善SH。NaOH浓度的降低增加了直接阳极氧化的作用,但减少了Fe(VI)的电化学生成。C 2 H 5 SH在灰铸铁阳极上在浓度较低的NaOH溶液中以较高的速率降解(在2 M NaOH溶液中k = 4.15×10 -2 min -1),这可以归因于直接阳极的增强氧化以及Fe(VI)的氧化能力。较高的电流密度促进了C 2 H 5的降解速率SH在直接和间接氧化过程中都可以,但是由于副反应的改善,它也降低了电流效率。此外,介绍和讨论了电化学生成的Fe(VI)在最近的研究中的现场和在线应用。这项研究提供了对灰铸铁阳极上C 2 H 5 SH氧化的全面理解,并提供了一种可行的电化学洗涤方法,使用灰铸铁作为阳极来控制C 2 H 5 SH气味。

































 京公网安备 11010802027423号
京公网安备 11010802027423号