Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-07-14 , DOI: 10.1016/j.bmc.2020.115622 Takamichi Imaizumi 1 , Shigeki Otsubo 2 , Masato Komai 2 , Hidenori Takada 3 , Michihiro Maemoto 1 , Atsuko Kobayashi 1 , Nobumasa Otsubo 1
|
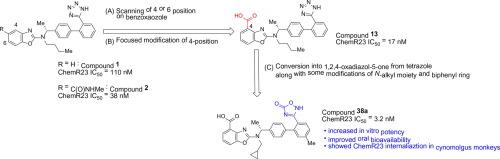
We previously reported 2-aminobenzoxazole analogue 1 as a potent ChemR23 inhibitor. The compound showed inhibitory activity against chemerin-induced calcium signaling through ChemR23 internalization in CAL-1 cells, which are cell lines of plasmacytoid dendric cells (pDCs). Furthermore, compound 2 inhibited chemotaxis of CAL-1 triggered by chemerin in vitro. However, we noted a difference in the ChemR23 response to our inhibitor between rodents and non-rodents in a previous study. To address this issue, we performed optimization of ChemR23 inhibitors using CAL-1 cells endogenously expressing human ChemR23 and conducted a pharmacokinetics study in cynomolgus monkeys. Various substituents at the 4-position of the benzoxazole ring exhibited potent in vitro bioactivity, while those at the 6-position were not tolerated. Among substituents, a carboxyl group was identified as key for improving the oral bioavailability in cynomolgus monkeys. Compound 38a with the acidic part changed from a tetrazole group to a 1,2,4-oxadiazol-5-one group to improve bioactivity and pharmacokinetic parameters exhibited inhibitory activity against chemerin-induced chemotaxis in vitro. In addition, we confirmed the ChemR23 internalization of pDCs by compound 38a orally administered to cynomolgus monkeys. These 2-aminobenzoxazole-based ChemR23 inhibitors may be useful as novel immunotherapeutic agents capable of suppressing the migration of pDCs, which are known to be major producers of type I interferons in the lesion area of certain autoimmune diseases, such as systemic lupus erythematosus and psoriasis.
中文翻译:

设计,合成和评估2-氨基苯并恶唑类似物作为有效和口服有效的ChemR23抑制剂。
我们先前曾报道过2-氨基苯并恶唑类似物1是有效的ChemR23抑制剂。该化合物在CAL-1细胞(浆细胞样树突状细胞(pDC))的Chem-1中通过ChemR23内化显示出针对凯莫瑞诱导的钙信号的抑制活性。此外,化合物2在体外抑制由凯莫瑞引发的CAL-1的趋化性。但是,我们注意到在先前的研究中,啮齿动物和非啮齿类动物对我们的抑制剂的ChemR23反应有所不同。为解决此问题,我们使用内源性表达人类ChemR23的CAL-1细胞对ChemR23抑制剂进行了优化,并在食蟹猴中进行了药代动力学研究。苯并恶唑环的4位上的各种取代基均显示出强大的体外生物活性,而在6-位的那些则不被容忍。在取代基中,羧基被认为是提高食蟹猴口服生物利用度的关键。化合物38a的酸性部分从四唑基变为1,2,4-恶二唑-5-基以改善生物活性,药代动力学参数对凯莫瑞诱导的趋化性具有抑制作用。此外,我们证实了化合物38a对pDC的ChemR23内在化食蟹猕猴口服。这些基于2-氨基苯并恶唑的ChemR23抑制剂可用作能够抑制pDC迁移的新型免疫治疗剂,已知这些pDC是某些自身免疫性疾病(例如系统性红斑狼疮和牛皮癣)病变区域的I型干扰素的主要生产者。 。































 京公网安备 11010802027423号
京公网安备 11010802027423号