当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-Pot Synthesis of 2,4-Diacyl Thiophenes from α-Oxo Ketene Dithioacetals and Propargylic Alcohols.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-07-13 , DOI: 10.1021/acs.joc.0c01093 Jian Xue 1 , Li-Gang Bai 1 , Liang Zhang 1 , Yue Zhou 1 , Xiao-Long Lin 1 , Neng-Jie Mou 1 , Dong-Rong Xiao 1 , Qun-Li Luo 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-07-13 , DOI: 10.1021/acs.joc.0c01093 Jian Xue 1 , Li-Gang Bai 1 , Liang Zhang 1 , Yue Zhou 1 , Xiao-Long Lin 1 , Neng-Jie Mou 1 , Dong-Rong Xiao 1 , Qun-Li Luo 1, 2
Affiliation
|
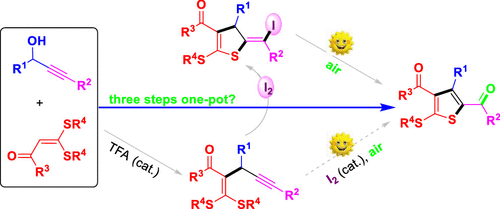
Although thiophenes having various functionalities are the basic structural units in numerous bioactive compounds and optoelectronic materials, synthetic routes to acylated thiophenes from aliphatic sulfur-containing starting materials are still rare. In particular, there have been no reports concerning the straightforward synthesis of 2,4-diacylthiophenes from alkynes. Herein, we describe a highly efficient and metal-free three-step one-pot synthetic approach to tetrasubstituted 2,4-diacylthiophenes from propargylic alcohols and α-oxo ketene dithioacetals. This research features a relay catalysis system that integrates Brønsted acid-catalyzed propargylation, molecular iodine-mediated electrophilic cyclization, and visible light-induced deiodinative oxygenation. The 2,4-diacylthiophenes serving as the key starting materials are readily synthesized, enabling facile construction of analogues of related biologically active compounds and the modular assembly of tetrasubstituted thienothiophenes.
中文翻译:

由α-氧代酮二硫缩醛和炔丙醇一锅法合成2,4-二酰基噻吩。
尽管具有多种功能的噻吩是许多生物活性化合物和光电材料中的基本结构单元,但是从脂肪族含硫原料到酰化噻吩的合成路线仍然很少。特别地,没有关于从炔烃直接合成2,4-二酰基噻吩的报道。在这里,我们描述了一种高效,无金属的三步法一锅法合成方法,用于从炔丙醇和α-氧代乙烯酮二硫缩醛中四取代的2,4-二酰基噻吩。这项研究的特点是一种中继催化系统,该系统集成了布朗斯台德酸催化的炔丙基化,分子碘介导的亲电环化和可见光诱导的脱碘氧合。用作关键原料的2,4-二乙酰基噻吩很容易合成,
更新日期:2020-08-08
中文翻译:

由α-氧代酮二硫缩醛和炔丙醇一锅法合成2,4-二酰基噻吩。
尽管具有多种功能的噻吩是许多生物活性化合物和光电材料中的基本结构单元,但是从脂肪族含硫原料到酰化噻吩的合成路线仍然很少。特别地,没有关于从炔烃直接合成2,4-二酰基噻吩的报道。在这里,我们描述了一种高效,无金属的三步法一锅法合成方法,用于从炔丙醇和α-氧代乙烯酮二硫缩醛中四取代的2,4-二酰基噻吩。这项研究的特点是一种中继催化系统,该系统集成了布朗斯台德酸催化的炔丙基化,分子碘介导的亲电环化和可见光诱导的脱碘氧合。用作关键原料的2,4-二乙酰基噻吩很容易合成,





















































 京公网安备 11010802027423号
京公网安备 11010802027423号