当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cosolvents in Self-Emulsifying Drug Delivery Systems (SEDDS): Do They Really Solve Our Solubility Problems?
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-07-13 , DOI: 10.1021/acs.molpharmaceut.0c00343 Arne Matteo Jörgensen 1 , Julian David Friedl 1 , Richard Wibel 1 , Joseph Chamieh 2 , Hervé Cottet 2 , Andreas Bernkop-Schnürch 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-07-13 , DOI: 10.1021/acs.molpharmaceut.0c00343 Arne Matteo Jörgensen 1 , Julian David Friedl 1 , Richard Wibel 1 , Joseph Chamieh 2 , Hervé Cottet 2 , Andreas Bernkop-Schnürch 1
Affiliation
|
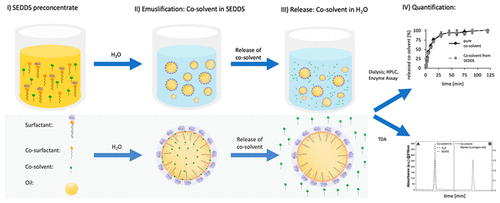
The aim of this study was to investigate the fate and the impact of cosolvents in self-emulsifying drug delivery systems (SEDDS). Three different SEDDS comprising the cosolvents DMSO (FD), ethanol (FE), and benzyl alcohol (FBA) as well as the corresponding formulations without these cosolvents (FD0, FE0, and FBA0) were developed. Mean droplet size, polydispersity index (PDI), ζ potential, stability, and emulsification time were determined. Cosolvent release studies were performed via the dialysis membrane method and Taylor dispersion analysis (TDA). Furthermore, the impact of cosolvent utilization on payloads in SEDDS was examined using quinine as a model drug. SEDDS with and without a cosolvent showed no significant differences in droplet size, PDI, and ζ potential. The emulsification time was 3-fold (FD0), 80-fold (FE0), and 7-fold (FBA0) longer due to the absence of the cosolvents. Release studies in demineralized water provided evidence for an immediate and complete release of DMSO, ethanol, and benzyl alcohol. TDA confirmed this result. Moreover, a 1.4-fold (FD), 2.91-fold (FE), and 2.17-fold (FBA) improved payload of the model drug quinine in the selected SEDDS preconcentrates was observed that dropped after emulsification within 1–5 h due to drug precipitation. In parallel, the quinine concentrations decreased until reaching the same levels of the corresponding SEDDS without cosolvents. Due to the addition of hydrophilic cosolvents, the emulsifying properties of SEDDS are strongly improved. As hydrophilic cosolvents are immediately released from SEDDS during the emulsification process, however, their drug solubilizing properties in the resulting oily droplets are very limited.
中文翻译:

自乳化药物递送系统(SEDDS)中的助溶剂:它们是否真的解决了我们的溶解性问题?
这项研究的目的是研究助溶剂在自乳化药物递送系统(SEDDS)中的命运和影响。包含助溶剂DMSO(F D),乙醇(F E)和苯甲醇(F BA)的三种不同的SEDDS以及不含这些助溶剂的相应配方(F D0,F E0和F BA0))开发。测定平均液滴尺寸,多分散指数(PDI),ζ电势,稳定性和乳化时间。通过透析膜方法和泰勒分散分析(TDA)进行助溶剂释放研究。此外,使用奎宁作为模型药物研究了助溶剂利用对SEDDS中有效负载的影响。使用和不使用助溶剂的SEDDS在液滴大小,PDI和ζ电位方面均无显着差异。由于不存在助溶剂,乳化时间延长了3倍(F D0),80倍(F E0)和7倍(F BA0)。在软化水中的释放研究为DMSO,乙醇和苯甲醇的立即完全释放提供了证据。TDA证实了这一结果。而且是1.4倍(FD),2.91倍(F E)和2.17倍(F BA)改善了所选SEDDS预浓缩物中模型药物奎宁的有效负载,由于药物沉淀,它们在乳化后1-5小时内下降。平行地,奎宁浓度降低直至达到没有助溶剂的相应SEDDS的相同水平。由于添加了亲水助溶剂,SEDDS的乳化性能得到了极大的改善。然而,由于亲水助溶剂在乳化过程中立即从SEDDS中释放出来,因此它们在所得油滴中的药物增溶性能非常有限。
更新日期:2020-09-09
中文翻译:

自乳化药物递送系统(SEDDS)中的助溶剂:它们是否真的解决了我们的溶解性问题?
这项研究的目的是研究助溶剂在自乳化药物递送系统(SEDDS)中的命运和影响。包含助溶剂DMSO(F D),乙醇(F E)和苯甲醇(F BA)的三种不同的SEDDS以及不含这些助溶剂的相应配方(F D0,F E0和F BA0))开发。测定平均液滴尺寸,多分散指数(PDI),ζ电势,稳定性和乳化时间。通过透析膜方法和泰勒分散分析(TDA)进行助溶剂释放研究。此外,使用奎宁作为模型药物研究了助溶剂利用对SEDDS中有效负载的影响。使用和不使用助溶剂的SEDDS在液滴大小,PDI和ζ电位方面均无显着差异。由于不存在助溶剂,乳化时间延长了3倍(F D0),80倍(F E0)和7倍(F BA0)。在软化水中的释放研究为DMSO,乙醇和苯甲醇的立即完全释放提供了证据。TDA证实了这一结果。而且是1.4倍(FD),2.91倍(F E)和2.17倍(F BA)改善了所选SEDDS预浓缩物中模型药物奎宁的有效负载,由于药物沉淀,它们在乳化后1-5小时内下降。平行地,奎宁浓度降低直至达到没有助溶剂的相应SEDDS的相同水平。由于添加了亲水助溶剂,SEDDS的乳化性能得到了极大的改善。然而,由于亲水助溶剂在乳化过程中立即从SEDDS中释放出来,因此它们在所得油滴中的药物增溶性能非常有限。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号