当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-Light Photocatalysis of Eosin Y: HAT and Complementing MS-CPET Strategy to Trifluoromethylation of β-Ketodithioesters with Langlois' Reagent.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-07-10 , DOI: 10.1021/acs.joc.0c01355
Sonam Soni 1 , Pragya Pali 1 , Monish Arbaz Ansari 1 , Maya Shankar Singh 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-07-10 , DOI: 10.1021/acs.joc.0c01355
Sonam Soni 1 , Pragya Pali 1 , Monish Arbaz Ansari 1 , Maya Shankar Singh 1
Affiliation
|
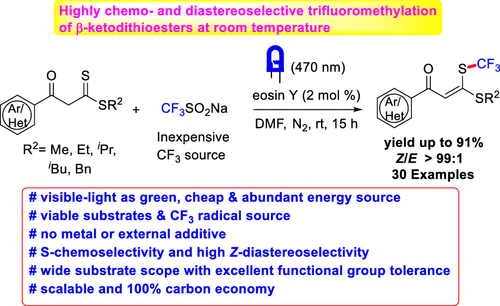
A metal- and oxidant-free photoinduced strategy for thioxo sulfur-selective trifluoromethylation of β-ketodithioesters at room temperature is reported. Excellent Z/E-stereoselectivity has been achieved with cheap and viable Langlois’ reagent (CF3SO2Na, sodium triflinate) in the presence of eosin Y, which acts as a hydrogen atom transfer (HAT) catalyst. The reaction proceeds via disulfide intermediate disulfanediylbis(3-(alkylthio)-1-phenylprop-2-en-1-one) (a dimer of β-ketodithioester) followed by complementing proton-coupled electron transfer-mediated reverse HAT cycle of eosin Y. This operationally simple and efficient protocol allows direct access to triflinated α-oxoketene dithioacetals in good to excellent yields bearing diverse synthetically useful functional groups of different electronic and steric nature.
中文翻译:

曙红Y:HAT的可见光光催化和使用Langlois试剂补充β-酮二硫代酯的三氟甲基化的MS-CPET策略。
报道了在室温下无金属和氧化剂的光诱导的β-酮二硫酯的硫代硫选择性三氟甲基化策略。在曙红Y的存在下,廉价和可行的Langlois试剂(CF 3 SO 2 Na,三氟甲磺酸钠)已经实现了出色的Z / E立体选择性,它起着氢原子转移(HAT)催化剂的作用。反应通过 二硫化物中间体二硫代二基双(3-(烷硫基)-1-苯基丙-2-烯-1-酮)(β-酮二硫酯的二聚体),然后补充曙红Y的质子偶联电子转移介导的反向HAT循环。这种操作简单高效且有效的方案可直接获得三氟代α-氧杂环丁烯二硫缩醛,且收率高至优异,带有带有不同电子和空间性质的各种合成有用的官能团。
更新日期:2020-08-08
中文翻译:

曙红Y:HAT的可见光光催化和使用Langlois试剂补充β-酮二硫代酯的三氟甲基化的MS-CPET策略。
报道了在室温下无金属和氧化剂的光诱导的β-酮二硫酯的硫代硫选择性三氟甲基化策略。在曙红Y的存在下,廉价和可行的Langlois试剂(CF 3 SO 2 Na,三氟甲磺酸钠)已经实现了出色的Z / E立体选择性,它起着氢原子转移(HAT)催化剂的作用。反应通过 二硫化物中间体二硫代二基双(3-(烷硫基)-1-苯基丙-2-烯-1-酮)(β-酮二硫酯的二聚体),然后补充曙红Y的质子偶联电子转移介导的反向HAT循环。这种操作简单高效且有效的方案可直接获得三氟代α-氧杂环丁烯二硫缩醛,且收率高至优异,带有带有不同电子和空间性质的各种合成有用的官能团。































 京公网安备 11010802027423号
京公网安备 11010802027423号