Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lipid-Dependent Interaction of Human N-BAR Domain Proteins with Sarcolemma Mono- and Bilayers.
Langmuir ( IF 3.7 ) Pub Date : 2020-07-10 , DOI: 10.1021/acs.langmuir.0c00649 Andrea Auerswald 1, 2 , Tobias Gruber 3 , Jochen Balbach 2, 3 , Annette Meister 1, 2
Langmuir ( IF 3.7 ) Pub Date : 2020-07-10 , DOI: 10.1021/acs.langmuir.0c00649 Andrea Auerswald 1, 2 , Tobias Gruber 3 , Jochen Balbach 2, 3 , Annette Meister 1, 2
Affiliation
|
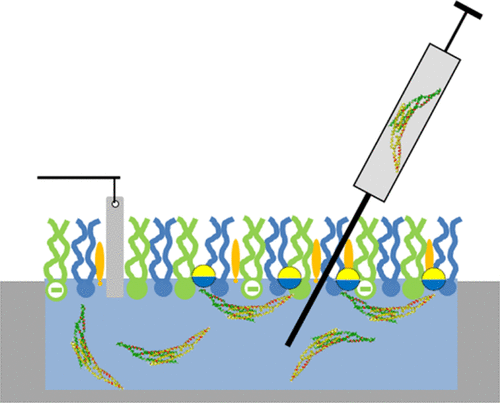
The N-BAR domain of the human Bin1 protein is indispensable for T-tubule biogenesis in skeletal muscles. It binds to lipid mono- and bilayers that mimic the sarcolemma membrane composition, and it transforms vesicles into uniform tubules by generating a decorating protein scaffold. We found that Δ(1–33)BAR, lacking the N-terminal amphipathic helix (H0), and H0 alone bind to sarcolemma monolayers, although both proteins are not able to tubulate sarcolemma vesicles. By variation of the lipid composition, we elucidated the role of PI(4,5)P2, cholesterol, and an asymmetric sarcolemma composition for Bin1-N-BAR binding and sarcolemma tubulation. Our results indicate that Bin1-N-BAR binding is low in the absence of PI(4,5)P2 and it is affected by additional changes in the negative headgroup charge and lipid acyl chain composition. However, it is not dependent on the cholesterol content. The results from Langmuir monolayer experiments are complementary to lipid bilayer studies using electron microscopy that provides information on membrane curvature generation.
中文翻译:

人N-BAR结构域蛋白与肉膜单层和双层的脂质依赖性相互作用。
人Bin1蛋白的N-BAR结构域对于骨骼肌中的T-管生物发生是必不可少的。它与模仿肌膜膜成分的脂质单层和双层结合,并通过产生装饰蛋白支架将囊泡转化为均匀的小管。我们发现,缺乏N末端两亲性螺旋(H0)的Δ(1-33)BAR和H0单独与肌膜单层结合,尽管这两种蛋白均不能使肌膜囊泡形成微管。通过改变脂质组成,我们阐明了PI(4,5)P 2,胆固醇和不对称肌膜组成对Bin1-N-BAR结合和肌膜管形成的作用。我们的结果表明,在没有PI(4,5)P 2的情况下,Bin1-N-BAR的结合很低并受到负头基电荷和脂质酰基链组成的其他变化的影响。但是,它不依赖于胆固醇含量。Langmuir单层实验的结果与使用电子显微镜的脂质双层研究相辅相成,后者提供了有关膜曲率产生的信息。
更新日期:2020-08-04
中文翻译:

人N-BAR结构域蛋白与肉膜单层和双层的脂质依赖性相互作用。
人Bin1蛋白的N-BAR结构域对于骨骼肌中的T-管生物发生是必不可少的。它与模仿肌膜膜成分的脂质单层和双层结合,并通过产生装饰蛋白支架将囊泡转化为均匀的小管。我们发现,缺乏N末端两亲性螺旋(H0)的Δ(1-33)BAR和H0单独与肌膜单层结合,尽管这两种蛋白均不能使肌膜囊泡形成微管。通过改变脂质组成,我们阐明了PI(4,5)P 2,胆固醇和不对称肌膜组成对Bin1-N-BAR结合和肌膜管形成的作用。我们的结果表明,在没有PI(4,5)P 2的情况下,Bin1-N-BAR的结合很低并受到负头基电荷和脂质酰基链组成的其他变化的影响。但是,它不依赖于胆固醇含量。Langmuir单层实验的结果与使用电子显微镜的脂质双层研究相辅相成,后者提供了有关膜曲率产生的信息。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号