Letters in Drug Design & Discovery ( IF 1.2 ) Pub Date : 2020-05-31 , DOI: 10.2174/1570180816666190731115809 Kancharla Suman 1 , Jyothi Prashanth 2 , Koya Prabhakara Rao 1 , Madala Subramanyam 1 , Vejendla Anuradha 1 , Mandava Venkata Basaveswara Rao 3
|
Background: Heterocyclic compounds containing heteroatoms (O, N and S) as part of five or six-membered cyclic moieties exhibited various potential applications, such as pharmaceutical drugs, agrochemical products and organic materials. Among many known heterocyclic moieties, quinoline and its derivatives are one of the privileged scaffolds found in many natural products. In general, quinoline derivatives could be prepared by utilizing ortho-substituted anilines and carbonyl compounds containing a reactive α-methylene group of well-known reaction routes like Friedlander synthesis, Niemantowski synthesis and Pfitzinger synthesis. Moreover, polysubstituted quinolones and their derivatives also had shown considerable interest in the fields of organic and pharmaceutical chemistry in recent years.
Objectives: The main objective of our research work is towards the design and synthesis of divergent biological-oriented, proactive analogues with potential pharmacological value inspired by the anti-tubercular activity of 2-phenylquinoline analogues. In this study, we have been interested in the design and synthesis of bioactive, 2, 4-diphenyl, 8-arylated quinoline analogues.
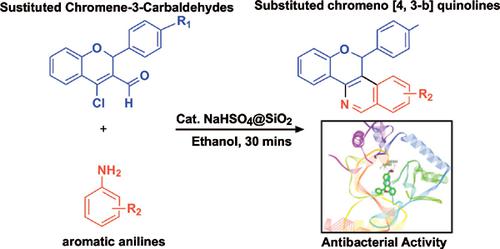
Methods: 6-phenyl-6h-chromeno [4, 3-b] quinoline derivatives were synthesized from 4-chloro-2- phenyl-2H-chromene-3-carbaldehyde and various substituted aromatic anilines as starting materials using sodium bisulfate embedded SiO2 re-usable catalyst. All these fifteen new compound structures confirmed by spectral data 1H & 13C NMR, Mass, CHN analysis etc. Furthermore, all these new compounds antibacterial activity strains recorded using the paper disc method. The compound molecular structures were designed using molecular docking study by utilizing the crystallographic parameters of S. Areus Murb protein.
Results: A series of fifteen new quinoline derivatives synthesized in moderate to good yields using sodium bisulfate embedded SiO2 re-usable catalyst. The molecular structures of these newly synthesized compounds elucidated by the combination of spectral data along with the elemental analysis. These compounds antibacterial activity study have shown moderate to good activity against, Escherichia coli (Gram-negative) and Staphylococcus aureus (gram-positive) organisms. These antibacterial activity results were also a very good correlation with molecular docking studies.
Conclusion: In this study, fifteen new quinoline derivatives synthesized and structures confirmed by spectral data. In fact, all the compounds have shown moderate to good antibacterial activity. In general, the compounds containing the electron donor group at R1 position (R1 = OMe) and the acceptor group at R2 positions (R2 = F or Cl) had shown good antibacterial activity. These antibacterial activity results were also a very good correlation with molecular docking studies showing strong binding energies with the highest value being, -12.45 Kcal mol-1 with S. aureus MurB receptor.
中文翻译:

NaHSO4 @ SiO2可重复使用催化剂轻松合成6-苯基-6h-chromeno [4,3-b]喹啉衍生物及其与分子对接研究的抗菌活性相关
背景:含有杂原子(O,N和S)作为五元或六元环状部分的杂环化合物具有多种潜在应用,例如药物,农用化学品和有机材料。在许多已知的杂环部分中,喹啉及其衍生物是在许多天然产物中发现的特有支架之一。通常,喹啉衍生物可以通过利用邻位取代的苯胺和含有反应性α-亚甲基的羰基化合物来制备,所述羰基化合物具有众所周知的反应路线,例如弗里德兰德合成,尼曼托夫斯基合成和普菲岑格合成。此外,近年来,多取代的喹诺酮及其衍生物在有机和药物化学领域也显示出相当大的兴趣。
目的:我们研究工作的主要目的是设计和合成具有不同药理价值的,具有潜在药理价值的,受2-苯基喹啉类似物抗结核活性启发的类似物。在这项研究中,我们一直对设计和合成具有生物活性的2,2,4-二苯基,8-芳基化喹啉类似物感兴趣。
方法:以硫酸氢钠包埋的SiO2为原料,以4-氯-2-苯基-2H-色烯-3-甲醛和各种取代的芳族苯胺为原料,合成6-苯基-6h-铬基[4,3-b]喹啉衍生物。 -可用的催化剂。通过光谱数据1H和13C NMR,质量,CHN分析等证实了所有这15种新化合物的结构。此外,所有这些新化合物的抗菌活性菌株均采用纸盘法记录。利用S. Areus Murb蛋白的晶体学参数,通过分子对接研究设计了化合物的分子结构。
结果:使用嵌入硫酸氢钠的SiO2可重复使用催化剂,以中等至良好的产率合成了15种新的喹啉衍生物。这些新合成的化合物的分子结构通过光谱数据与元素分析的结合得以阐明。这些化合物的抗菌活性研究表明,它们对大肠杆菌(革兰氏阴性)和金黄色葡萄球菌(革兰氏阳性)生物具有中等至良好的活性。这些抗菌活性结果也与分子对接研究非常相关。
结论:在这项研究中,合成了十五种新的喹啉衍生物,并通过光谱数据证实了结构。实际上,所有化合物均显示出中等至良好的抗菌活性。通常,在R1位置含有电子给体基团(R1 = OMe)而在R2位置含有受体基团(R2 = F或Cl)的化合物表现出良好的抗菌活性。这些抗菌活性结果也与分子对接研究具有很好的相关性,分子对接研究显示与金黄色葡萄球菌MurB受体的结合能最高,值为-12.45 Kcal mol-1。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号